当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic and kinetics study on the reaction of CF3CBrCH2 with OH: A theoretical study
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-07-09 , DOI: 10.1002/poc.4079 Yunju Zhang 1 , Yuxi Sun 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-07-09 , DOI: 10.1002/poc.4079 Yunju Zhang 1 , Yuxi Sun 1
Affiliation
|
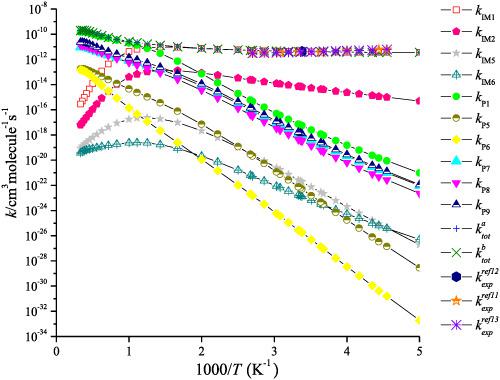
Quantum chemical method (CCSD(T)/cc‐pVTZ//M06‐2X/6‐311++G(d,p)) is employed to research the CF3CBrCH2 + OH reaction. The results indicate that the reaction takes place through the interaction of the oxygen atom of the OH radical with the middle C and terminal C atom of CF3CBrCH2 generating adduct IM1 (CF3CBrCH2OH) and IM2 (CF3CBrOHCH2), respectively, and then further dissociation or rearrangement to many products. The rate constants have been computed at 10−10 to 1010 Torr and 200–3000 K by RRKM theory for various product pathways. The results show that at 200–800 K, the rate constant for the production of IM1 (CF3CBrCH2OH) by collisional deactivation is dominant; at high temperatures, the production of P1 (CF3CBrCHOH + H) becomes predominate. The predicted data for CF3CBrCH2 + OH agree closely with available experimental value. The total rate constants are independent on pressure and dependent on temperature. The rate equation can be fitted as k(T) = 1.77 × 10 −7T−0.65exp(−4518.77/T) at 200–300 K, 30 Torr of Ar. The atmospheric lifetime of CF3CBrCH2 in OH is around 2.77 days. TD‐DFT computations imply that IM1, IM2, IM3, IM4, IM5, and IM6 will photolyze under the sunlight.
中文翻译:

CF3CBrCH2与OH反应的机理和动力学研究:理论研究
量子化学方法(CCSD(T)/ CC-pVTZ // M06-2X / 6-311 ++ G(d,ρ))被用来研究对CF 3 CBrCH 2 + OH反应。结果表明,反应发生通过自由基与CF的中央C和终端C原子的OH的氧原子的相互作用3 CBrCH 2生成加合物IM1(CF 3 CBrCH 2 OH)和IM2(CF 3 CBrOHCH 2),然后进一步分解或重排为许多产品。速率常数已计算为10 -10至10 10根据RRKM理论,Torr和200–3000 K适用于各种产品路径。结果表明,在200–800 K时,碰撞失活产生IM1(CF 3 CBrCH 2 OH)的速率常数占优势;在高温下,P1(CF 3 CBrCHOH + H)的产生占主导地位。对于CF预测数据3 CBrCH 2 + OH与实验值紧密一致。总速率常数与压力无关,与温度无关。速率方程可以拟合为k(T)= 1.77×10 -7 T -0.65 exp(-4518.77 / T)在200–300 K,30 Torr的Ar。CF的大气寿命3 CBrCH 2中OH是围绕2.77天。TD-DFT计算表明IM1,IM2,IM3,IM4,IM5和IM6将在阳光下发生光解。
更新日期:2020-09-11
中文翻译:

CF3CBrCH2与OH反应的机理和动力学研究:理论研究
量子化学方法(CCSD(T)/ CC-pVTZ // M06-2X / 6-311 ++ G(d,ρ))被用来研究对CF 3 CBrCH 2 + OH反应。结果表明,反应发生通过自由基与CF的中央C和终端C原子的OH的氧原子的相互作用3 CBrCH 2生成加合物IM1(CF 3 CBrCH 2 OH)和IM2(CF 3 CBrOHCH 2),然后进一步分解或重排为许多产品。速率常数已计算为10 -10至10 10根据RRKM理论,Torr和200–3000 K适用于各种产品路径。结果表明,在200–800 K时,碰撞失活产生IM1(CF 3 CBrCH 2 OH)的速率常数占优势;在高温下,P1(CF 3 CBrCHOH + H)的产生占主导地位。对于CF预测数据3 CBrCH 2 + OH与实验值紧密一致。总速率常数与压力无关,与温度无关。速率方程可以拟合为k(T)= 1.77×10 -7 T -0.65 exp(-4518.77 / T)在200–300 K,30 Torr的Ar。CF的大气寿命3 CBrCH 2中OH是围绕2.77天。TD-DFT计算表明IM1,IM2,IM3,IM4,IM5和IM6将在阳光下发生光解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号