当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent inhibition profiles and inverse solvent isotope effects for enzymatic methyl transfer catalyzed by nicotinamide N‐methyltransferase
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-06-30 , DOI: 10.1002/poc.4093 Yuping Li 1 , Yali Zhang 1 , Yiting Cheng 1 , Tianshu Du 1 , Jianyu Zhang 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-06-30 , DOI: 10.1002/poc.4093 Yuping Li 1 , Yali Zhang 1 , Yiting Cheng 1 , Tianshu Du 1 , Jianyu Zhang 1
Affiliation
|
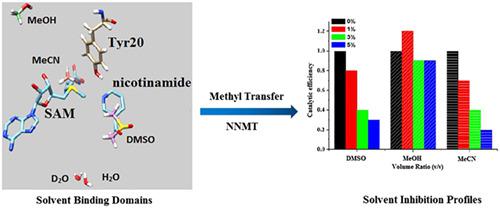
Nicotinamide N‐methyltransferase (NNMT) catalyzes the methyl transfer from universal methyl donor S‐adenosyl methionine (SAM) to nicotinamide (NA) to form the N‐methylnicotinamide. This important process is related to the level of nicotinamide adenine dinucleotide (NAD+) in vivo; thus, NNMT is regarded as an important drug target linked with various diseases. Although NNMT has been extensively studied in the area of biology and medicine, the detailed mechanism of NNMT is still not clear, especially in the aspect of the role of solvents (water) in the enzyme catalysis. Here, we have examined the effects of three common organic solvents (dimethyl sulfoxide [DMSO], methanol, and acetonitrile) and deuterated water to explore the enzymatic methyl transfer activity by NNMT (wild type [WT]) and its mutants. Competitive inhibition was detected for DMSO and acetonitrile as the solvent, and a subtle inhibitory effect was observed with methanol. Molecular docking suggested DMSO and acetonitrile compete with both cofactor and substrate. However, methanol is mainly bound to compete for the substrate. Furthermore, the activity increased when deuterated water was substituted for water with an inverse solvent isotope effect D2Okcat/Km = 0.49 ± 0.17 for WT and D2Okcat/Km = 0.32 ± 0.08 for Y20F. These effects in this enzymatic methyl transfer system without any metal ion or acid/base catalysis were due to the stabilization of protein provided by deuterated water.
中文翻译:

烟酰胺N-甲基转移酶催化的酶促甲基转移的溶剂抑制特征和溶剂同位素反作用
烟酰胺N-甲基转移酶(NNMT)催化从通用甲基供体S-腺苷甲硫氨酸(SAM)到烟酰胺(NA)的甲基转移,形成N-甲基烟酰胺。这个重要过程与烟酰胺腺嘌呤二核苷酸(NAD +)体内;因此,NNMT被认为是与多种疾病相关的重要药物靶标。尽管在生物学和医学领域已经对NNMT进行了广泛的研究,但NNMT的详细机理仍不清楚,尤其是在溶剂(水)在酶催化中的作用方面。在这里,我们检查了三种常见的有机溶剂(二甲基亚砜[DMSO],甲醇和乙腈)和氘水的作用,以探索NNMT(野生型[WT])及其突变体的酶促甲基转移活性。检测到对DMSO和乙腈为溶剂的竞争性抑制作用,并且观察到甲醇具有微妙的抑制作用。分子对接表明DMSO和乙腈与辅因子和底物竞争。但是,甲醇必定会竞争底物。此外,对于WT,D 2 k k cat / K m= 0.49±0.17,对于Y20F ,D 2 O k cat / K m= 0.32±0.08。在没有任何金属离子或酸/碱催化的情况下,这种酶促甲基转移体系中的这些作用是由于氘化水提供的蛋白质的稳定性。
更新日期:2020-06-30
中文翻译:

烟酰胺N-甲基转移酶催化的酶促甲基转移的溶剂抑制特征和溶剂同位素反作用
烟酰胺N-甲基转移酶(NNMT)催化从通用甲基供体S-腺苷甲硫氨酸(SAM)到烟酰胺(NA)的甲基转移,形成N-甲基烟酰胺。这个重要过程与烟酰胺腺嘌呤二核苷酸(NAD +)体内;因此,NNMT被认为是与多种疾病相关的重要药物靶标。尽管在生物学和医学领域已经对NNMT进行了广泛的研究,但NNMT的详细机理仍不清楚,尤其是在溶剂(水)在酶催化中的作用方面。在这里,我们检查了三种常见的有机溶剂(二甲基亚砜[DMSO],甲醇和乙腈)和氘水的作用,以探索NNMT(野生型[WT])及其突变体的酶促甲基转移活性。检测到对DMSO和乙腈为溶剂的竞争性抑制作用,并且观察到甲醇具有微妙的抑制作用。分子对接表明DMSO和乙腈与辅因子和底物竞争。但是,甲醇必定会竞争底物。此外,对于WT,D 2 k k cat / K m= 0.49±0.17,对于Y20F ,D 2 O k cat / K m= 0.32±0.08。在没有任何金属离子或酸/碱催化的情况下,这种酶促甲基转移体系中的这些作用是由于氘化水提供的蛋白质的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号