当前位置:
X-MOL 学术
›
Acta Cryst. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the crystal structures and phase transitions of hydrates in the binary dimethyl sulfoxide–water system
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2020-08-20 , DOI: 10.1107/s2052520620008999 A. D. Fortes , J. Ponsonby , O. Kirichek , V. García-Sakai
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2020-08-20 , DOI: 10.1107/s2052520620008999 A. D. Fortes , J. Ponsonby , O. Kirichek , V. García-Sakai
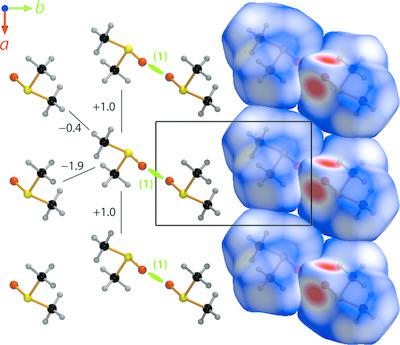
|
Neutron powder diffraction data have been collected from a series of flash‐frozen aqueous solutions of dimethyl sulfoxide (DMSO) with concentrations between 25 and 66.7 mol% DMSO. These reveal the existence of three stoichiometric hydrates, which crystallize on warming between 175 and 195 K. DMSO trihydrate crystallizes in the monoclinic space group P21/c, with unit‐cell parameters at 195 K of a = 10.26619 (3), b = 7.01113 (2), c = 10.06897 (3) Å, β = 101.5030 (2)° and V = 710.183 (3) Å3 (Z = 4). Two of the symmetry‐inequivalent water molecules form a sheet of tiled four‐ and eight‐sided rings; the DMSO molecules are sandwiched between these sheets and linked along the b axis by the third water molecule to generate water–DMSO–water tapes. Two different polymorphs of DMSO dihydrate have been identified. The α phase is monoclinic (space group P21/c), with unit‐cell parameters at 175 K of a = 6.30304 (4), b = 9.05700 (5), c = 11.22013 (7) Å, β = 105.9691 (4)° and V = 615.802 (4) Å3 (Z = 4). Its structure contains water–DMSO–water chains, but these are polymerized in such a manner as to form sheets of reniform eight‐sided rings, with the methyl groups extending on either side of the sheet. On warming above 198 K, α‐DMSO·2H2O undergoes a solid‐state transformation to a mixture of DMSO·3H2O + anhydrous DMSO, and there is then a stable eutectic between these two phases at ∼203 K. The β‐phase of DMSO dihydrate has been observed in a rapidly frozen eutectic melt and in very DMSO‐rich mixtures. It is observed to be unstable with respect to the α‐phase; above ∼180 K, β‐DMSO·2H2O converts irreversibly to α‐DMSO·2H2O. At 175 K, the lattice parameters of β‐DMSO·2H2O are a = 6.17448 (10), b = 11.61635 (16), c = 8.66530 (12) Å, β = 101.663 (1)° and V = 608.684 (10) Å3 (Z = 4), hence this polymorph is just 1.16% denser than the α‐phase under identical conditions. Like the other two hydrates, the space group appears likely, on the basis of systematic absences, to be P21/c, but the structure has not yet been determined. Our results reconcile 60 years of contradictory interpretations of the phase relations in the binary DMSO–water system, particularly between mole fractions of 0.25–0.50, and confirm empirical and theoretical studies of the liquid structure around the eutectic composition (33.33 mol% DMSO).
中文翻译:

关于二甲基二甲亚砜-水系统中水合物的晶体结构和相变
中子粉衍射数据是从一系列二甲基亚砜(DMSO)的速冻水溶液中收集的,DMSO浓度在25至66.7 mol%之间。这些揭示了三种化学计量的水合物的存在,它们在175至195 K的升温中结晶。DMSO三水合物在单斜空间群P 2 1 / c中结晶,在195 K时的晶胞参数a = 10.26619(3),b = 7.01113(2),C ^ = 10.06897(3),β= 101.5030(2)°,V = 710.183(3)3(ž= 4)。两个不对称的水分子形成一块平铺的四面和八面环;DMSO分子夹在这些薄片之间,并通过第三个水分子沿b轴连接,生成水-DMSO-水带。DMSO二水合物有两种不同的多晶型物。α相为单斜晶(空间群P 2 1 / c),175 K时的晶胞参数为a = 6.30304(4),b = 9.05700(5),c = 11.22013(7)Å,β= 105.9691( 4)°,V = 615.802(4)3(ž= 4)。它的结构包含水-DMSO-水链,但这些链以某种方式聚合形成了肾形的八边环片,甲基在片的两侧延伸。当温度升高到198 K以上时,α-DMSO·2H 2 O发生固态转变为DMSO·3H 2 O +无水DMSO的混合物,然后这两个相之间在〜203 K处存在稳定的共晶。在快速冷冻的共晶熔体和非常富DMSO的混合物中观察到DMSO二水合物的相。观察到它相对于α相是不稳定的。以上〜180 K,β-DMSO·2H 2 ö不可逆地转换到α-DMSO·2H 2 O.在175 K,β-DMSO的晶格参数·2H 2的O一= 6.17448(10),b = 11.61635(16),C ^ = 8.66530(12)α,β= 101.663(1)°,V = 608.684(10)一种3(Ž = 4),因此,此多晶型物是只是1.16%在相同条件下比α相致密。像其他两个水合物一样,在系统性缺席的基础上,空间群似乎可能为P 2 1 / c,但结构尚未确定。我们的结果调和了对二元DMSO-水系统中相关系的60年矛盾解释,特别是0.25-0.50的摩尔分数之间的相交,并证实了对共晶成分(33.33 mol%DMSO)周围液体结构的经验和理论研究。
更新日期:2020-10-07
中文翻译:

关于二甲基二甲亚砜-水系统中水合物的晶体结构和相变
中子粉衍射数据是从一系列二甲基亚砜(DMSO)的速冻水溶液中收集的,DMSO浓度在25至66.7 mol%之间。这些揭示了三种化学计量的水合物的存在,它们在175至195 K的升温中结晶。DMSO三水合物在单斜空间群P 2 1 / c中结晶,在195 K时的晶胞参数a = 10.26619(3),b = 7.01113(2),C ^ = 10.06897(3),β= 101.5030(2)°,V = 710.183(3)3(ž= 4)。两个不对称的水分子形成一块平铺的四面和八面环;DMSO分子夹在这些薄片之间,并通过第三个水分子沿b轴连接,生成水-DMSO-水带。DMSO二水合物有两种不同的多晶型物。α相为单斜晶(空间群P 2 1 / c),175 K时的晶胞参数为a = 6.30304(4),b = 9.05700(5),c = 11.22013(7)Å,β= 105.9691( 4)°,V = 615.802(4)3(ž= 4)。它的结构包含水-DMSO-水链,但这些链以某种方式聚合形成了肾形的八边环片,甲基在片的两侧延伸。当温度升高到198 K以上时,α-DMSO·2H 2 O发生固态转变为DMSO·3H 2 O +无水DMSO的混合物,然后这两个相之间在〜203 K处存在稳定的共晶。在快速冷冻的共晶熔体和非常富DMSO的混合物中观察到DMSO二水合物的相。观察到它相对于α相是不稳定的。以上〜180 K,β-DMSO·2H 2 ö不可逆地转换到α-DMSO·2H 2 O.在175 K,β-DMSO的晶格参数·2H 2的O一= 6.17448(10),b = 11.61635(16),C ^ = 8.66530(12)α,β= 101.663(1)°,V = 608.684(10)一种3(Ž = 4),因此,此多晶型物是只是1.16%在相同条件下比α相致密。像其他两个水合物一样,在系统性缺席的基础上,空间群似乎可能为P 2 1 / c,但结构尚未确定。我们的结果调和了对二元DMSO-水系统中相关系的60年矛盾解释,特别是0.25-0.50的摩尔分数之间的相交,并证实了对共晶成分(33.33 mol%DMSO)周围液体结构的经验和理论研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号