当前位置:
X-MOL 学术
›
J. Synchrotron Radiat.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In situ structural studies during denaturation of natural and synthetically crosslinked collagen using synchrotron SAXS.
Journal of Synchrotron Radiation ( IF 2.5 ) Pub Date : 2020-08-19 , DOI: 10.1107/s1600577520009479 Yi Zhang , Jenna Buchanan , Rafea Naffa , Bradley Mansel , Catherine Maidment , Geoff Holmes , Sujay Prabakar
Journal of Synchrotron Radiation ( IF 2.5 ) Pub Date : 2020-08-19 , DOI: 10.1107/s1600577520009479 Yi Zhang , Jenna Buchanan , Rafea Naffa , Bradley Mansel , Catherine Maidment , Geoff Holmes , Sujay Prabakar
|
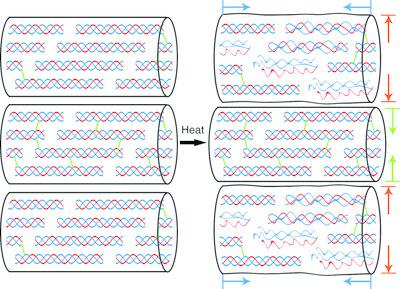
Collagen is an important biomacromolecule, making up the majority of the extracellular matrix in animal tissues. Naturally occurring crosslinks in collagen stabilize its intermolecular structure in vivo, whereas chemical treatments for introducing synthetic crosslinks are often carried out ex vivo to improve the physical properties or heat stability of the collagen fibres for applications in biomaterials or leather production. Effective protection of intrinsic natural crosslinks as well as allowing them to contribute to collagen stability together with synthetic crosslinks can reduce the need for chemical treatments. However, the contribution of these natural crosslinks to the heat stability of collagen fibres, especially in the presence of synthetic crosslinks, is as yet unknown. Using synchrotron small‐angle X‐ray scattering, the in situ role of natural and synthetic crosslinks on the stabilization of the intermolecular structure of collagen in skins was studied. The results showed that, although natural crosslinks affected the denaturation temperature of collagen, they were largely weakened when crosslinked using chromium sulfate. The development of synergistic crosslinking chemistries could help retain the intrinsic chemical and physical properties of collagen‐based biological materials.
中文翻译:

使用同步加速器SAXS在天然和合成交联胶原蛋白变性过程中的原位结构研究。
胶原蛋白是重要的生物大分子,占动物组织中细胞外基质的大部分。胶原蛋白中天然存在的交联键可在体内稳定其分子间结构,而引入合成交联键的化学处理通常在离体时进行以改善用于生物材料或皮革生产的胶原纤维的物理性能或热稳定性。有效保护固有的天然交联键以及使其与合成交联键一起有助于胶原蛋白的稳定性,可以减少化学处理的需要。然而,这些天然交联对胶原纤维的热稳定性的贡献,特别是在存在合成交联的情况下,仍是未知的。使用同步加速器小角度X射线散射,原位研究了天然和合成交联在稳定皮肤胶原蛋白分子间结构中的作用。结果表明,尽管天然交联会影响胶原的变性温度,但是当使用硫酸铬交联时,它们会大大减弱。协同交联化学的发展可以帮助保留基于胶原的生物材料的固有化学和物理特性。
更新日期:2020-08-19
中文翻译:

使用同步加速器SAXS在天然和合成交联胶原蛋白变性过程中的原位结构研究。
胶原蛋白是重要的生物大分子,占动物组织中细胞外基质的大部分。胶原蛋白中天然存在的交联键可在体内稳定其分子间结构,而引入合成交联键的化学处理通常在离体时进行以改善用于生物材料或皮革生产的胶原纤维的物理性能或热稳定性。有效保护固有的天然交联键以及使其与合成交联键一起有助于胶原蛋白的稳定性,可以减少化学处理的需要。然而,这些天然交联对胶原纤维的热稳定性的贡献,特别是在存在合成交联的情况下,仍是未知的。使用同步加速器小角度X射线散射,原位研究了天然和合成交联在稳定皮肤胶原蛋白分子间结构中的作用。结果表明,尽管天然交联会影响胶原的变性温度,但是当使用硫酸铬交联时,它们会大大减弱。协同交联化学的发展可以帮助保留基于胶原的生物材料的固有化学和物理特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号