当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational design of Fe2(μ-PR2)2(L)6 coordination compounds featuring tailored potential inversion.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-08-20 , DOI: 10.1002/cphc.202000623 Federica Arrigoni 1 , Fabio Rizza 1 , Jacopo Vertemara 1 , Raffaella Breglia 2 , Claudio Greco 2 , Luca Bertini 1 , Giuseppe Zampella 1 , Luca De Gioia 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-08-20 , DOI: 10.1002/cphc.202000623 Federica Arrigoni 1 , Fabio Rizza 1 , Jacopo Vertemara 1 , Raffaella Breglia 2 , Claudio Greco 2 , Luca Bertini 1 , Giuseppe Zampella 1 , Luca De Gioia 1
Affiliation
|
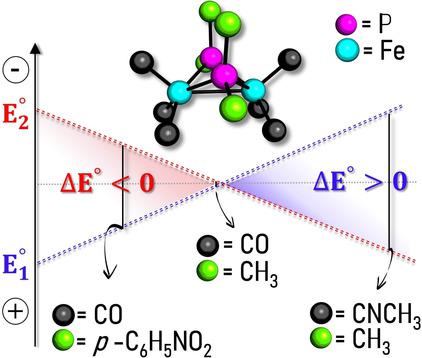
It was recently discovered that some redox proteins can thermodynamically and spatially split two incoming electrons towards different pathways, resulting in the one‐electron reduction of two different substrates, featuring reduction potential respectively higher and lower than the parent reductant. This energy conversion process, referred to as electron bifurcation, is relevant not only from a biochemical perspective, but also for the ground‐breaking applications that electron‐bifurcating molecular devices could have in the field of energy conversion. Natural electron‐bifurcating systems contain a two‐electron redox centre featuring potential inversion (PI), i. e. with second reduction easier than the first. With the aim of revealing key factors to tailor the span between first and second redox potentials, we performed a systematic density functional study of a 26‐molecule set of models with the general formula Fe2(μ‐PR2)2(L)6. It turned out that specific features such as i) a Fe−Fe antibonding character of the LUMO, ii) presence of electron‐donor groups and iii) low steric congestion in the Fe's coordination sphere, are key ingredients for PI. In particular, the synergic effects of i)‐iii) can lead to a span between first and second redox potentials larger than 700 mV. More generally, the “molecular recipes” herein described are expected to inspire the synthesis of Fe2P2 systems with tailored PI, of primary relevance to the design of electron‐bifurcating molecular devices.
中文翻译:

Fe2(μ-PR2)2(L)6配位化合物的量身定制的潜在反演的合理设计。
最近发现,某些氧化还原蛋白可以将两个传入的电子在热力学和空间上朝着不同的途径分裂,从而导致两种不同底物的单电子还原,其还原电位分别高于或低于母体还原剂。这种能量转换过程被称为电子分叉,不仅从生化角度来看,而且与电子分叉分子器件在能量转换领域可能具有的突破性应用有关。天然电子分叉系统包含一个具有电位反转(PI)的双电子氧化还原中心,即。e。第二个还原比第一个还原容易。为了揭示关键因素以调整第一和第二氧化还原电势之间的跨度,2(μ‐PR 2)2(L)6。事实证明,一些特殊特征是PI的关键成分,这些特征包括:i)LUMO的Fe-Fe反键特性,ii)电子给体基团的存在以及iii)Fe配位球的低位阻。特别是,i)-iii)的协同作用会导致第一和第二氧化还原电势之间的跨度大于700 mV。更一般而言,本文所述的“分子配方”有望激发具有定制PI的Fe 2 P 2体系的合成,这与电子分叉分子器件的设计主要相关。
更新日期:2020-10-19
中文翻译:

Fe2(μ-PR2)2(L)6配位化合物的量身定制的潜在反演的合理设计。
最近发现,某些氧化还原蛋白可以将两个传入的电子在热力学和空间上朝着不同的途径分裂,从而导致两种不同底物的单电子还原,其还原电位分别高于或低于母体还原剂。这种能量转换过程被称为电子分叉,不仅从生化角度来看,而且与电子分叉分子器件在能量转换领域可能具有的突破性应用有关。天然电子分叉系统包含一个具有电位反转(PI)的双电子氧化还原中心,即。e。第二个还原比第一个还原容易。为了揭示关键因素以调整第一和第二氧化还原电势之间的跨度,2(μ‐PR 2)2(L)6。事实证明,一些特殊特征是PI的关键成分,这些特征包括:i)LUMO的Fe-Fe反键特性,ii)电子给体基团的存在以及iii)Fe配位球的低位阻。特别是,i)-iii)的协同作用会导致第一和第二氧化还原电势之间的跨度大于700 mV。更一般而言,本文所述的“分子配方”有望激发具有定制PI的Fe 2 P 2体系的合成,这与电子分叉分子器件的设计主要相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号