当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heterologous production and biochemical characterization of a new highly glucose tolerant GH1 β-glucosidase from Anoxybacillus thermarum
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.procbio.2020.08.013 Paula Zaghetto de Almeida , Tássio Brito de Oliveira , Rosymar Coutinho de Lucas , José Carlos Santos Salgado , Malena Martínez Pérez , Beatriz Gálan , José Luis García , Maria de Lourdes Teixeira de Moraes Polizeli
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.procbio.2020.08.013 Paula Zaghetto de Almeida , Tássio Brito de Oliveira , Rosymar Coutinho de Lucas , José Carlos Santos Salgado , Malena Martínez Pérez , Beatriz Gálan , José Luis García , Maria de Lourdes Teixeira de Moraes Polizeli
|
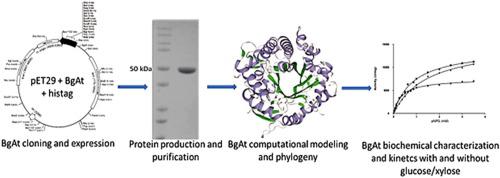
Abstract The enzymatic lignocellulosic biomass conversion into value-added products requires the use of enzyme-rich cocktails, including β-glucosidases that hydrolyze cellobiose and cellooligosaccharides to glucose. During hydrolysis occurs accumulation of monomers causing inhibition of some enzymes; thus, glucose/xylose tolerant β-glucosidases could overcome this drawback. The search of new tolerant enzymes showing additional properties, such as high activity, wide-pH range, and thermal stability is very relevant to improve the bioprocess. We describe a novel β-glucosidase GH1 from the thermophilic Anoxybacillus thermarum (BgAt), which stood out by the robustness combination of great glucose/xylose tolerance, thermal stability, and high Vmax. The recombinant his-tagged-BgAt was overexpressed in Escherichia coli, was purified in one step, showed a high glucose/xylose tolerance, and activity stimulation (presence of 0.4 M glucose/1.0 M xylose). The optimal activity was at 65 °C - pH 7.0. BgAt presented an extraordinary temperature stability (48 h – 50 °C), and pH stability (5.5–8.0). The novel enzyme showed outstanding Vmax values compared to other β-glucosidases. Using p-nitrophenyl-β- d -glucopyranoside as substrate the values were Vmax (7614 U/mg), and KM (0.360 mM). These values suffer a displacement in Vmax to 14,026 U/mg (glucose), 14,886 U/mg (xylose), and KM 0.877 mM (glucose), and 1.410 mM (xylose).
中文翻译:

来自 Anoxybacillus thermarum 的新型高葡萄糖耐受性 GH1 β-葡萄糖苷酶的异源生产和生化表征
摘要 酶促木质纤维素生物质转化为增值产品需要使用富含酶的混合物,包括将纤维二糖和纤维寡糖水解成葡萄糖的 β-葡萄糖苷酶。在水解过程中发生单体的积累,导致一些酶的抑制;因此,葡萄糖/木糖耐受性 β-葡萄糖苷酶可以克服这个缺点。寻找具有额外特性(例如高活性、宽 pH 范围和热稳定性)的新耐受酶与改进生物过程非常相关。我们描述了一种来自嗜热无氧芽孢杆菌 (BgAt) 的新型 β-葡萄糖苷酶 GH1,它具有出色的葡萄糖/木糖耐受性、热稳定性和高 Vmax 的稳健性组合。重组his-tagged-BgAt在大肠杆菌中过表达,一步纯化,显示出高葡萄糖/木糖耐受性和活动刺激(存在 0.4 M 葡萄糖/1.0 M 木糖)。最佳活性为 65 °C - pH 7.0。BgAt 表现出非凡的温度稳定性 (48 h – 50 °C) 和 pH 稳定性 (5.5–8.0)。与其他 β-葡萄糖苷酶相比,这种新型酶显示出出色的 Vmax 值。使用对硝基苯基-β-d-吡喃葡萄糖苷作为底物,值是 Vmax (7614 U/mg) 和 KM (0.360 mM)。这些值的 Vmax 位移为 14,026 U/mg(葡萄糖)、14,886 U/mg(木糖)和 KM 0.877 mM(葡萄糖)和 1.410 mM(木糖)。与其他 β-葡萄糖苷酶相比,这种新型酶显示出出色的 Vmax 值。使用对硝基苯基-β-d-吡喃葡萄糖苷作为底物,值是 Vmax (7614 U/mg) 和 KM (0.360 mM)。这些值的 Vmax 位移为 14,026 U/mg(葡萄糖)、14,886 U/mg(木糖)和 KM 0.877 mM(葡萄糖)和 1.410 mM(木糖)。与其他 β-葡萄糖苷酶相比,这种新型酶显示出出色的 Vmax 值。使用对硝基苯基-β-d-吡喃葡萄糖苷作为底物,值是 Vmax (7614 U/mg) 和 KM (0.360 mM)。这些值的 Vmax 位移为 14,026 U/mg(葡萄糖)、14,886 U/mg(木糖)和 KM 0.877 mM(葡萄糖)和 1.410 mM(木糖)。
更新日期:2020-12-01
中文翻译:

来自 Anoxybacillus thermarum 的新型高葡萄糖耐受性 GH1 β-葡萄糖苷酶的异源生产和生化表征
摘要 酶促木质纤维素生物质转化为增值产品需要使用富含酶的混合物,包括将纤维二糖和纤维寡糖水解成葡萄糖的 β-葡萄糖苷酶。在水解过程中发生单体的积累,导致一些酶的抑制;因此,葡萄糖/木糖耐受性 β-葡萄糖苷酶可以克服这个缺点。寻找具有额外特性(例如高活性、宽 pH 范围和热稳定性)的新耐受酶与改进生物过程非常相关。我们描述了一种来自嗜热无氧芽孢杆菌 (BgAt) 的新型 β-葡萄糖苷酶 GH1,它具有出色的葡萄糖/木糖耐受性、热稳定性和高 Vmax 的稳健性组合。重组his-tagged-BgAt在大肠杆菌中过表达,一步纯化,显示出高葡萄糖/木糖耐受性和活动刺激(存在 0.4 M 葡萄糖/1.0 M 木糖)。最佳活性为 65 °C - pH 7.0。BgAt 表现出非凡的温度稳定性 (48 h – 50 °C) 和 pH 稳定性 (5.5–8.0)。与其他 β-葡萄糖苷酶相比,这种新型酶显示出出色的 Vmax 值。使用对硝基苯基-β-d-吡喃葡萄糖苷作为底物,值是 Vmax (7614 U/mg) 和 KM (0.360 mM)。这些值的 Vmax 位移为 14,026 U/mg(葡萄糖)、14,886 U/mg(木糖)和 KM 0.877 mM(葡萄糖)和 1.410 mM(木糖)。与其他 β-葡萄糖苷酶相比,这种新型酶显示出出色的 Vmax 值。使用对硝基苯基-β-d-吡喃葡萄糖苷作为底物,值是 Vmax (7614 U/mg) 和 KM (0.360 mM)。这些值的 Vmax 位移为 14,026 U/mg(葡萄糖)、14,886 U/mg(木糖)和 KM 0.877 mM(葡萄糖)和 1.410 mM(木糖)。与其他 β-葡萄糖苷酶相比,这种新型酶显示出出色的 Vmax 值。使用对硝基苯基-β-d-吡喃葡萄糖苷作为底物,值是 Vmax (7614 U/mg) 和 KM (0.360 mM)。这些值的 Vmax 位移为 14,026 U/mg(葡萄糖)、14,886 U/mg(木糖)和 KM 0.877 mM(葡萄糖)和 1.410 mM(木糖)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号