Nitric Oxide ( IF 3.2 ) Pub Date : 2020-08-19 , DOI: 10.1016/j.niox.2020.08.002 Huixian Ye 1 , Hailing Li 2 , Zhonghong Gao 2
|
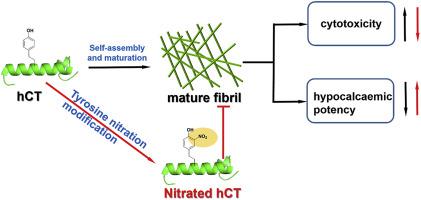
Irreversible aggregation can extremely limit the bioavailability and therapeutic activity of peptide-based drugs. There is therefore an urgent demand of effective strategy to control peptide aggregation. Recently, we found that tyrosine nitration at certain sites of peptide can effectively inhibit its aggregation. This minor modification may be an ideal strategy to the rational design of peptide-based drugs with low aggregation propensity yet without loss of bioactivity. Human calcitonin (hCT) is such a peptide hormone known for its hypocalcaemic effect but has limited pharmaceutical potential due to a high tendency to aggregate. In this study, by using multiple techniques including Fluorescence, TEM, Nu-PAGE and CD, we demonstrated that Y12 nitration of hCT would significantly inhibit its self-assembles, and we also found that this modification would not only reduce the cytotoxicity induced by peptide aggregation, but also had little effect on its potency. This finding may provide a novel strategy for clinically application of hCT instead of sCT.
中文翻译:

降钙素(hCT)的Y12硝化:产生非聚集生物活性hCT的一种有前途的策略。
不可逆的聚集会极大地限制基于肽的药物的生物利用度和治疗活性。因此,迫切需要有效的策略来控制肽的聚集。最近,我们发现在肽的某些位点酪氨酸硝化可以有效抑制其聚集。这种小的修饰可能是合理设计具有低聚集倾向但又不损失生物活性的基于肽的药物的理想策略。降钙素(hCT)是这种肽激素,因其降钙作用而闻名,但由于高度聚集的趋势而具有有限的药物开发潜力。在这项研究中,通过使用多种技术,包括荧光技术,TEM,Nu-PAGE和CD,我们证明了hCT的Y12硝化将显着抑制其自组装,并且我们还发现,这种修饰不仅会减少由肽聚集引起的细胞毒性,而且对其效力几乎没有影响。这一发现可能为临床应用hCT代替sCT提供了一种新颖的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号