Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2020-08-20 , DOI: 10.1016/j.omtm.2020.08.012 Malte Lenders , Franciska Stappers , Eva Brand
|
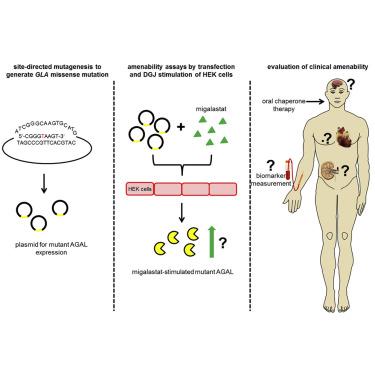
Migalastat (1-deoxygalactonojirimycin) is approved for the treatment of Fabry disease (FD) in patients with an amenable mutation. Currently, there are at least 367 amenable and 711 non-amenable mutations known, based on an in vitro good laboratory practice (GLP) assay. Recent studies demonstrated that in vitro amenability of mutations did not necessarily correspond to in vivo amenability of migalastat-treated patients. This discrepancy might be due to (methodological) limitations of the current GLP-HEK assay. Currently, there are several published comparable cell-based amenability assays, with partially different outcomes for the same tested mutation, leading to concerns in FD-treating physicians. The aim of this review is to elucidate the idea of amenability assays from their beginning, starting with patient-specific primary cells to high-throughput assays based on overexpression. Consequently, we compare methods of current assays, highlighting their similarities, as well as their pros and cons. Finally, we provide a literature-based list of α-galactosidase A mutations, tested by different assays to provide a comprehensive overview of amenable mutations as a good basis for the decision-making by treating physicians. Since in vitro amenability does not always correspond with in vivo amenability, the treating clinician has the responsibility to monitor clinical and laboratory features to verify clinical response.
中文翻译:

法布里病中米格司他的体外和体内适应性
Migalastat(1-deoxygalactonojirimycin)被批准用于治疗具有适当突变的患者的Fabry病(FD)。当前,基于体外实验室质量管理规范(GLP)分析,已知至少有367个顺应性突变和711个不顺应性突变。最近的研究表明,突变的体外适应性不一定与体内的相符接受米加司他治疗的患者的适应性。这种差异可能是由于当前GLP-HEK分析的(方法)局限性引起的。当前,有几种已发表的基于细胞的可比性可比性测定方法,对于相同的测试突变,其结果部分不同,这引起了FD治疗医生的关注。这篇综述的目的是阐明适应性测定的想法,从适合患者的原代细胞到基于过表达的高通量测定开始。因此,我们比较了当前检测方法,突出了它们的相似性以及它们的优缺点。最后,我们提供了基于文献的α-半乳糖苷酶A突变列表,并通过不同的测试方法对其进行了测试,以提供对可适应突变的全面概述,作为治疗医师做出决策的良好基础。体外适应性并不总是与体内适应性相对应,主治临床医生有责任监测临床和实验室特征以验证临床反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号