当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
What decides the kinetics of V2+/V3+ and VO2+/VO2+ redox reactions – Surface functional groups or roughness?
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jelechem.2020.114590 Pradipkumar Leuaa , Divya Priyadarshani , Anand Kumar Tripathi , Manoj Neergat
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jelechem.2020.114590 Pradipkumar Leuaa , Divya Priyadarshani , Anand Kumar Tripathi , Manoj Neergat
|
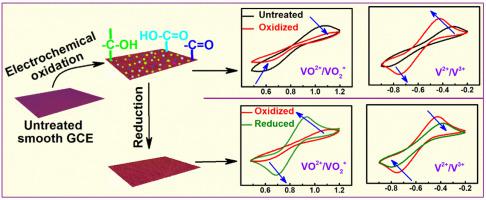
Abstract Carbon paper (CP) electrodes are electrochemically treated in 1 M H2SO4 for various time durations to functionalize their surface with oxygenated groups. Such electrodes are subsequently used to investigate the kinetics of redox reactions relevant to the vanadium redox flow batteries (VRFBs). The results suggest that the oxidized electrodes catalyse the kinetics of V2+/V3+ reaction and impede that of the VO2+/VO2+ reaction. On cycling the electrode to lower potentials (−0.25 to −0.9 V), relevant to the negative electrode of VRFB, the V2+/V3+ reaction kinetics decelerates. Yet, the reduced electrodes show improved kinetics than that of the untreated carbon electrodes. On the other hand, the VO2+/VO2+ reaction kinetics is improved, as compared to that of the untreated carbon electrodes. The origin of such interesting and yet contradicting results are explained using XPS and Zeta-3D imaging of the oxidized and reduced carbon electrodes. The role of oxygenated functional groups and surface roughness of the electrode on the redox reaction kinetics of vanadium ions is established.
中文翻译:

什么决定了 V2+/V3+ 和 VO2+/VO2+ 氧化还原反应的动力学——表面官能团或粗糙度?
摘要 复写纸 (CP) 电极在 1 M H2SO4 中经过不同时间的电化学处理,以使其表面具有含氧基团功能化。这些电极随后用于研究与钒氧化还原液流电池 (VRFB) 相关的氧化还原反应动力学。结果表明,氧化电极催化了 V2+/V3+ 反应的动力学并阻碍了 VO2+/VO2+ 反应的动力学。在将电极循环至与 VRFB 负极相关的较低电位(-0.25 至 -0.9 V)时,V2+/V3+ 反应动力学减速。然而,与未处理的碳电极相比,还原电极显示出改进的动力学。另一方面,与未经处理的碳电极相比,VO2+/VO2+ 反应动力学得到改善。使用氧化和还原碳电极的 XPS 和 Zeta-3D 成像解释了这种有趣但又相互矛盾的结果的起源。建立了氧化官能团和电极表面粗糙度对钒离子氧化还原反应动力学的作用。
更新日期:2020-12-01
中文翻译:

什么决定了 V2+/V3+ 和 VO2+/VO2+ 氧化还原反应的动力学——表面官能团或粗糙度?
摘要 复写纸 (CP) 电极在 1 M H2SO4 中经过不同时间的电化学处理,以使其表面具有含氧基团功能化。这些电极随后用于研究与钒氧化还原液流电池 (VRFB) 相关的氧化还原反应动力学。结果表明,氧化电极催化了 V2+/V3+ 反应的动力学并阻碍了 VO2+/VO2+ 反应的动力学。在将电极循环至与 VRFB 负极相关的较低电位(-0.25 至 -0.9 V)时,V2+/V3+ 反应动力学减速。然而,与未处理的碳电极相比,还原电极显示出改进的动力学。另一方面,与未经处理的碳电极相比,VO2+/VO2+ 反应动力学得到改善。使用氧化和还原碳电极的 XPS 和 Zeta-3D 成像解释了这种有趣但又相互矛盾的结果的起源。建立了氧化官能团和电极表面粗糙度对钒离子氧化还原反应动力学的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号