Cell Calcium ( IF 4.3 ) Pub Date : 2020-08-20 , DOI: 10.1016/j.ceca.2020.102256 Romane Idoux 1 , Clarisse Fuster 1 , Vincent Jacquemond 1 , Anamika Dayal 2 , Manfred Grabner 2 , Pierre Charnet 3 , Bruno Allard 1
|
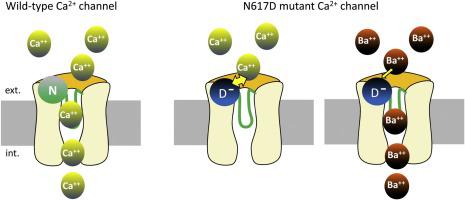
In response to excitation of skeletal muscle fibers, trains of action potentials induce changes in the configuration of the dihydropyridine receptor (DHPR) anchored in the tubular membrane which opens the Ca2+ release channel in the sarcoplasmic reticulum membrane. The DHPR also functions as a voltage-gated Ca2+ channel that conducts L-type Ca2+ currents routinely recorded in mammalian muscle fibers, which role was debated for more than four decades. Recently, to allow a closer look into the role of DHPR Ca2+ influx in mammalian muscle, a knock-in (ki) mouse model (ncDHPR) carrying mutation N617D (adjacent to domain II selectivity filter E) in the DHPRα1S subunit abolishing Ca2+ permeation through the channel was generated [Dayal et al., 2017]. In the present study, the Mn2+ quenching technique was initially intended to be used on voltage-clamped muscle fibers from this mouse to determine whether Ca2+ influx through a pathway distinct from DHPR may occur to compensate for the absence of DHPR Ca2+ influx. Surprisingly, while N617D DHPR muscle fibers of the ki mouse do not conduct Ca2+, Mn2+ entry and subsequent quenching did occur because Mn2+ was able to permeate and produce L-type currents through N617D DHPR. N617D DHPR was also found to conduct Ba2+ and Ba2+ currents were strongly blocked by external Ca2+. Ba2+ permeation was smaller, current kinetics slower and Ca2+ block more potent than in wild-type DHPR. These results indicate that residue N617 when replaced by the negatively charged residue D is suitably located at entrance of the pore to trap external Ca2+ impeding in this way permeation. Because Ba2+ binds with lower affinity to D, Ba2+ currents occur, but with reduced amplitudes as compared to Ba2+ currents through wild-type channels. We conclude that mutations located outside the selectivity filter influence channel permeation and possibly channel gating in a fully differentiated skeletal muscle environment.
中文翻译:

Ca2+ 非导电骨骼肌二氢吡啶受体小鼠模型中的二价阳离子渗透。
响应于骨骼肌纤维的激发,一系列动作电位诱导固定在管状膜中的二氢吡啶受体 (DHPR) 的构型发生变化,从而打开肌质网膜中的 Ca 2+释放通道。DHPR 还充当电压门控 Ca 2+通道,其传导在哺乳动物肌肉纤维中常规记录的L 型 Ca 2+电流,其作用已争论了四个多十年。最近,为了更深入地研究 DHPR Ca 2+流入在哺乳动物肌肉中的作用,在 DHPRα 1S 中携带突变 N617D(与域 II 选择性过滤器 E 相邻)的敲入 ( ki ) 小鼠模型 ( nc DHPR)产生了通过通道消除 Ca 2+渗透的亚基[Dayal et al., 2017]。在本研究中,Mn 2+淬火技术最初旨在用于该小鼠的电压钳位肌纤维,以确定 Ca 2+是否可能通过与 DHPR 不同的途径流入,以补偿 DHPR Ca 2的缺失+涌入。令人惊讶的是,虽然ki小鼠的N617D DHPR 肌纤维不传导 Ca 2+,但确实发生了Mn 2+进入和随后的淬灭,因为 Mn 2+能够渗透并通过 N617D DHPR 产生 L 型电流。N617D DHPR 也被发现传导 Ba 2+Ba 2+电流被外部Ca 2+强烈阻断。与野生型 DHPR 相比,Ba 2+渗透更小,电流动力学更慢,Ca 2+阻断更有效。这些结果表明,残基 N617 当被带负电荷的残基 D 取代时适合位于孔的入口处以捕获以这种方式阻碍渗透的外部 Ca 2+。因为 Ba 2+与 D 的亲和力较低,所以会出现Ba 2+电流,但与 Ba 2+相比振幅降低通过野生型通道的电流。我们得出结论,位于选择性过滤器之外的突变会影响完全分化的骨骼肌环境中的通道渗透和可能的通道门控。











































 京公网安备 11010802027423号
京公网安备 11010802027423号