Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-08-19 , DOI: 10.1016/j.bmcl.2020.127509 Samer S Daher 1 , Kevin P Franklin 1 , Tyler Scherzi 2 , Paul M Dunman 2 , Rodrigo B Andrade 1
|
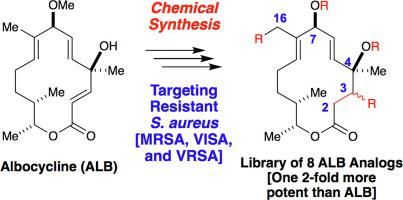
Albocycline (ALB) is a unique macrolactone natural product with potent, narrow-spectrum activity against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate (VISA), and vancomycin-resistant S. aureus (VRSA) strains (MIC = 0.5–1.0 μg/mL). Described herein is the synthesis and evaluation of a novel series analogs derived from albocycline by functionalization at three specific sites: the C2-C3 enone, the tertiary carbinol at C4, and the allylic C16 methyl group. Exploration of the structure-activity relationships (SAR) by means of minimum inhibitory concentration assays (MICs) revealed that C4 ester analog 6 was twice as potent as ALB, which represents a class of lead compound that can be further studied to address multi-drug resistant pathogens.
中文翻译:

半合成白环素类似物的合成和生物学评价。
Albocycline (ALB) 是一种独特的大环内酯天然产物,对耐甲氧西林金黄色葡萄球菌(MRSA)、万古霉素中间体 (VISA) 和耐万古霉素金黄色葡萄球菌(VRSA) 菌株具有强效、窄谱活性(MIC = 0.5– 1.0 微克/毫升)。本文描述了通过在三个特定位点官能化而衍生自白环素的新型系列类似物的合成和评价:C2-C3 烯酮、C4 上的叔甲醇和烯丙基 C16 甲基。通过最小抑制浓度测定 (MIC) 对构效关系 (SAR) 的探索表明 C4 酯类似物6 ALB 的效力是 ALB 的两倍,后者代表了一类可以进一步研究以解决多重耐药病原体的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号