Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-08-20 , DOI: 10.1016/j.bmc.2020.115716 Andrey A Ivashchenko 1 , Yan A Ivanenkov 2 , Vladimir A Aladinskiy 3 , Ruben N Karapetian 4 , Angela G Koryakova 4 , Alexey A Ryakhovskiy 4 , Oleg D Mitkin 4 , Dmitry V Kravchenko 4 , Nikolai P Savchuk 5 , Bogdan A Zagribelnyy 3 , Alexander V Ivashchenko 6
|
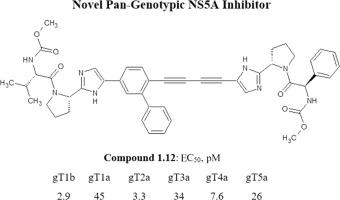
A series of novel small-molecule pan-genotypic hepatitis C virus (HCV) NS5A inhibitors with picomolar activity containing 2-[(2S)-pyrrolidin-2-yl]-5-[4-(4-{2-[(2S)-pyrrolidin-2-yl]-1H-imidazol-5-yl}buta-1,3-diyn-1-yl)phenyl]-1H-imidazole core was designed based on molecular modeling study and SAR analysis. The constructed in silico model and docking study provide a deep insight into the binding mode of this type of NS5A inhibitors. Based on the predicted binding interface we have prioritized the most crucial diversity points responsible for improving antiviral activity. The synthesized molecules were tested in a cell-based assay, and compound 1.12 showed an EC50 value in the range of 2.9-34 pM against six genotypes of NS5A HCV, including gT3a, and demonstrated favorable pharmacokinetic profile in rats. This lead compound can be considered as an attractive candidate for further clinical evaluation.
中文翻译:

新型泛基因型 NS5A 抑制剂的合成、生物学评价和计算机模拟
一系列具有皮摩尔活性的新型小分子泛基因型丙型肝炎病毒 (HCV) NS5A 抑制剂,含有 2-[(2 S )-pyrrolidin-2-yl]-5-[4-(4-{2-[( 2 S )-pyrrolidin-2-yl]-1 H -imidazol-5-yl}buta-1,3-diyn-1-yl)phenyl]-1 H -imidazole core 基于分子模型研究和SAR分析设计. 构建的计算机模型和对接研究提供了对此类 NS5A 抑制剂结合模式的深入了解。基于预测的结合界面,我们优先考虑了负责提高抗病毒活性的最关键的多样性点。合成的分子在基于细胞的测定中进行了测试,化合物1.12显示出 EC 50对六种 NS5A HCV 基因型(包括 gT3a)的检测值在 2.9-34 pM 范围内,并在大鼠中显示出良好的药代动力学特征。这种先导化合物可以被认为是进一步临床评估的有吸引力的候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号