Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2020-08-20 , DOI: 10.1016/j.abb.2020.108547 Suet Y Lo 1 , Danica L Goulet 1 , Usama Fraaz 1 , Stefan Siemann 1
|
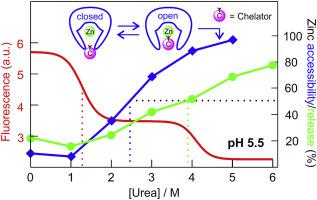
Anthrax lethal factor (LF) is a critical component of the anthrax toxin, and functions intracellularly as a zinc-dependent endopeptidase targeting proteins involved in maintaining critical host signaling pathways. To reach the cytoplasm, LF requires to be unfolded and guided through the narrow protective antigen pore in a pH-dependent process. The current study sought to address the question as to whether LF is capable of retaining its metal ion when exposed to a low-pH environment (similar to that found in late endosomes) and an unfolding stress (induced by urea). Using a combination of tryptophan fluorescence spectroscopy and chelation studies, we show that a decrease in the pH value (from 7.0 to 5.0) leads to a pronounced shift in the onset of structural alterations in LF to lower urea concentrations. More importantly, the enzyme was found to retain its Zn2+ ion beyond the unfolding transitions monitored by Trp fluorescence, a finding indicative of tight metal binding to LF in a non-native state. In addition, an analysis of red-edge excitation shift (REES) spectra suggests the protein to maintain residual structure (a feature necessary for metal binding) even at very high denaturant concentrations. Furthermore, studies using the chromophoric chelator 4-(2-pyridylazo)resorcinol (PAR) revealed LF's Zn2+ ion to become accessible to complexation at urea concentrations in between those required to cause structural changes and metal dissociation. This phenomenon likely originates from the conversion of a PAR-inaccessible (closed) to a PAR-accessible (open) state of LF at intermediate denaturant concentrations.
中文翻译:

pH和变性剂对炭疽致死因子折叠和金属状态的影响。
炭疽致死因子(LF)是炭疽毒素的重要组成部分,在细胞内起着锌依赖性内肽酶靶向蛋白的作用,参与维持关键宿主信号通路。为了到达细胞质,需要在依赖于pH的过程中将LF展开并引导通过狭窄的保护性抗原孔。当前的研究试图解决以下问题:LF在暴露于低pH环境(类似于晚期内体中发现的环境)和展开应力(由尿素引起)时是否能够保留其金属离子。结合使用色氨酸荧光光谱法和螯合研究,我们发现pH值的降低(从7.0到5.0)导致LF的结构改变开始时尿素浓度明显降低。更重要的是,在Trp荧光监测下,超过2+离子的未折叠转变表明,紧密的金属以非天然状态结合到LF。此外,对红边激发位移(REES)光谱的分析表明,即使在非常高的变性剂浓度下,该蛋白质也能保持残留结构(金属结合所必需的特征)。此外,使用发色螯合剂4-(2-吡啶基偶氮)间苯二酚(PAR)进行的研究显示,在尿素浓度介于LF的Zn 2+离子之间,络合可使其络合,从而引起结构变化和金属解离。此现象可能是由于在中间变性剂浓度下,LF的PAR不可访问(封闭)状态转换为LF的PAR可访问(开放)状态引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号