当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The synthesis of new acyclic analogs of 3-phenacyluridine and comparative evaluation of their in vivo biological activity
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-07-18 , DOI: 10.1007/s10593-020-02729-x Ivan А. Novakov , Leila L. Brunilina , Ivan А. Kirillov , Maxim B. Nawrozkij , Mariya D. Robinovich , Evgeniya S. Titova , Dmitry S. Sheikin , Evsey А. Ruchko , Alla V. Pavlova , Anastasiya А. Kotlyarova , Tatyana G. Tolstikova

中文翻译:

新的3-苯并吡啶核苷无环类似物的合成及其体内生物活性的比较评价

Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-07-18 , DOI: 10.1007/s10593-020-02729-x Ivan А. Novakov , Leila L. Brunilina , Ivan А. Kirillov , Maxim B. Nawrozkij , Mariya D. Robinovich , Evgeniya S. Titova , Dmitry S. Sheikin , Evsey А. Ruchko , Alla V. Pavlova , Anastasiya А. Kotlyarova , Tatyana G. Tolstikova
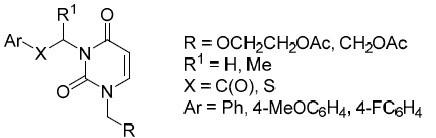
|

中文翻译:

新的3-苯并吡啶核苷无环类似物的合成及其体内生物活性的比较评价












































 京公网安备 11010802027423号
京公网安备 11010802027423号