当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-hydrogen bond catalyst-mediated diastereoselective conjugate additions of 5H-oxazol-4-ones to o-hydroxyphenyl-substituted p-quinone methides.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-08-19 , DOI: 10.1039/d0ob01558j Ziyang Wang 1 , Anqi Huang , Fang Fang , Pengfei Li , Guokai Liu , Wenjun Li
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-08-19 , DOI: 10.1039/d0ob01558j Ziyang Wang 1 , Anqi Huang , Fang Fang , Pengfei Li , Guokai Liu , Wenjun Li
Affiliation
|
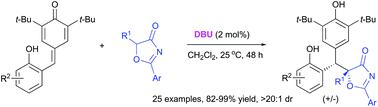
An efficient DBU-catalyzed conjugate addition of 5H-oxazol-4-ones to o-hydroxyphenyl-substituted p-quinone methides has been developed, affording the valuable diarylmethanes in high yields with excellent diastereoselectivity. This strategy demonstrates a robust access to a wide range of diarylmethane derivatives possessing biologically significant o-hydroxyphenol and p-hydroxyphenol moieties under mild reaction conditions.
中文翻译:

非氢键催化剂介导的 5H-恶唑-4-酮与邻羟基苯基取代的对醌甲基化物的非对映选择性共轭加成。
开发了一种高效的 DBU 催化共轭加成 5 H-恶唑-4-酮与邻羟基苯基取代的对醌甲基化物,以高收率提供有价值的二芳基甲烷,并具有出色的非对映选择性。该策略表明,在温和的反应条件下,可以稳健地获得具有生物学意义的邻羟基苯酚和对羟基苯酚部分的各种二芳基甲烷衍生物。
更新日期:2020-09-16
中文翻译:

非氢键催化剂介导的 5H-恶唑-4-酮与邻羟基苯基取代的对醌甲基化物的非对映选择性共轭加成。
开发了一种高效的 DBU 催化共轭加成 5 H-恶唑-4-酮与邻羟基苯基取代的对醌甲基化物,以高收率提供有价值的二芳基甲烷,并具有出色的非对映选择性。该策略表明,在温和的反应条件下,可以稳健地获得具有生物学意义的邻羟基苯酚和对羟基苯酚部分的各种二芳基甲烷衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号