当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inclusion of viologen cations leads to switchable metal–organic frameworks
Faraday Discussions ( IF 3.4 ) Pub Date : 2020-08-19 , DOI: 10.1039/c9fd00137a Laura K. Cadman 1, 2, 3, 4 , Mary F. Mahon 1, 2, 3, 4 , Andrew D. Burrows 1, 2, 3, 4
Faraday Discussions ( IF 3.4 ) Pub Date : 2020-08-19 , DOI: 10.1039/c9fd00137a Laura K. Cadman 1, 2, 3, 4 , Mary F. Mahon 1, 2, 3, 4 , Andrew D. Burrows 1, 2, 3, 4
Affiliation
|
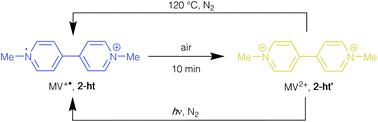
The reactions of Zn(NO3)2·6H2O with the polycarboxylic acids 1,3-benzenedicarboxylic acid (H2mbdc), 1,4-benzenedicarboxylic acid (H2bdc), 1,3,5-benzenetricarboxylic acid (H3btc) and 4,4′-biphenyldicarboxylic acid (H2bpdc) in the presence of methyl viologen iodide ([MV]I2) in DMF gave anionic frameworks with methyl viologen species incorporated as counter-ions. When the reactions were carried out at 120 °C, the blue products [MV][Zn3(mbdc)4] (1-ht), [MV]0.44[H2MV]0.36[NMe2H2]0.4[Zn3(bdc)4]·0.6DMF (2-ht), [MV]0.5[Zn(btc)]·DMF (4-ht) and [MV][Zn4(bpdc)5]·8DMF·10H2O (5-ht) were formed, and these were shown to contain the radical cation [MV]˙+. In contrast, the same reactions carried out at 85 °C gave orange isostructural compounds containing the dication [MV]2+. Similar observations were made for reactions with ethyl viologen bromide. The compounds 1-ht, 2-ht and 4-ht contain similar framework topologies to analogues in which NMe2H2+ is the included cation. In contrast, 5-ht is based on a previously unreported interpenetrated network. Compound 2-ht contains the protonated species [H2MV]2+ in addition to [MV]˙+ and the crystal structure shows that the two rings in the former are staggered with respect to each other. This species is believed to form under the reaction conditions employed in the synthesis and the formation of [H2MV]2+ is suppressed by using an alternative approach in which methyl viologen is formed in situ from viologen diacetic acid. In the bdc-containing products, the radical cation is rapidly oxidised to the dication on exposure to air, as witnessed by the colour change from blue to orange. This change is reversed either by heating to 120 °C or exposure to UV radiation, both under nitrogen. This is in contrast to observations with the mbdc and btc analogues 1-ht and 4-ht, as in these compounds the blue colour persists for weeks. The difference can be related to the structures, with the channels present in 2-ht allowing oxygen to reach the radical cations.
中文翻译:

包含紫罗兰色阳离子导致可切换的金属有机框架
的Zn(NO的反应3)2 ·6H 2 O,其多元羧酸-1,3-苯二甲酸(H 2 MBDC),1,4-苯二羧酸(H 2 BDC),1,3,5-苯三甲酸(在DMF中存在甲基碘化碘([MV] I 2)的条件下,H 3 btc)和4,4'-联苯二甲酸(H 2 bpdc)产生了阴离子骨架,其中甲基紫精物质作为抗衡离子被引入。当反应在120°C进行时,蓝色产物[MV] [Zn 3(mbdc)4 ](1-ht),[MV] 0.44 [H 2 MV] 0.36 [NMe2 H 2 ] 0.4 [Zn 3(bdc) 4 ]·0.6DMF( 2-ht),[MV] 0.5 [Zn(btc)]·DMF( 4-ht)和[MV] [Zn 4(bpdc) 5形成了]·8DMF·10H 2 O( 5-ht),并显示它们含有自由基阳离子[MV]˙ +。相比之下,在85°C进行的相同反应得到的橙色同构化合物含有[MV] 2+指示。对于与乙基紫精溴的反应也有类似的观察。化合物1-ht, 2-ht和4-ht包含与NMe 2 H 2 +是所含阳离子的类似物相似的骨架拓扑。相比之下,5-ht基于先前未报告的互穿网络。化合物2-ht除[MV] +外还含有质子化的物质[H 2 MV] 2+,晶体结构显示前者中的两个环彼此交错。该物质被认为是在合成中使用的反应条件下形成的,并且通过使用其中原位形成甲基紫精的另一种方法抑制了[H 2 MV] 2+的形成。来自紫精二乙酸。在含bdc的产品中,自由基阳离子在暴露于空气后会迅速氧化成指示剂,从蓝色到橙色的颜色变化可以证明这一点。通过在氮气下加热到120°C或暴露于紫外线辐射,可以逆转这种变化。这与mbdc和btc类似物1-ht和4-ht的观察结果相反,因为在这些化合物中,蓝色持续数周。差异可能与结构有关,通道中存在2 ht的通道,使氧气可以到达自由基阳离子。
更新日期:2020-08-19
中文翻译:

包含紫罗兰色阳离子导致可切换的金属有机框架
的Zn(NO的反应3)2 ·6H 2 O,其多元羧酸-1,3-苯二甲酸(H 2 MBDC),1,4-苯二羧酸(H 2 BDC),1,3,5-苯三甲酸(在DMF中存在甲基碘化碘([MV] I 2)的条件下,H 3 btc)和4,4'-联苯二甲酸(H 2 bpdc)产生了阴离子骨架,其中甲基紫精物质作为抗衡离子被引入。当反应在120°C进行时,蓝色产物[MV] [Zn 3(mbdc)4 ](1-ht),[MV] 0.44 [H 2 MV] 0.36 [NMe2 H 2 ] 0.4 [Zn 3(bdc) 4 ]·0.6DMF( 2-ht),[MV] 0.5 [Zn(btc)]·DMF( 4-ht)和[MV] [Zn 4(bpdc) 5形成了]·8DMF·10H 2 O( 5-ht),并显示它们含有自由基阳离子[MV]˙ +。相比之下,在85°C进行的相同反应得到的橙色同构化合物含有[MV] 2+指示。对于与乙基紫精溴的反应也有类似的观察。化合物1-ht, 2-ht和4-ht包含与NMe 2 H 2 +是所含阳离子的类似物相似的骨架拓扑。相比之下,5-ht基于先前未报告的互穿网络。化合物2-ht除[MV] +外还含有质子化的物质[H 2 MV] 2+,晶体结构显示前者中的两个环彼此交错。该物质被认为是在合成中使用的反应条件下形成的,并且通过使用其中原位形成甲基紫精的另一种方法抑制了[H 2 MV] 2+的形成。来自紫精二乙酸。在含bdc的产品中,自由基阳离子在暴露于空气后会迅速氧化成指示剂,从蓝色到橙色的颜色变化可以证明这一点。通过在氮气下加热到120°C或暴露于紫外线辐射,可以逆转这种变化。这与mbdc和btc类似物1-ht和4-ht的观察结果相反,因为在这些化合物中,蓝色持续数周。差异可能与结构有关,通道中存在2 ht的通道,使氧气可以到达自由基阳离子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号