Letters in Organic Chemistry ( IF 0.7 ) Pub Date : 2020-07-31 , DOI: 10.2174/1570178617666200207111127 Zetryana Puteri Tachrim 1 , Kazuhiro Oida 1 , Fumina Ohashi 1 , Natsumi Kurokawa 1 , Lei Wang 2 , Takeyuki Suzuki 3 , Makoto Hashimoto 1
|
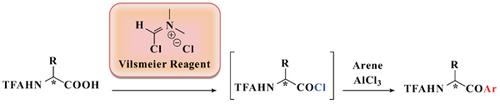
α-Amino acid chlorides are reactive coupling agents in amide (peptide) formation. The Vilsmeier reagent ((chloromethylene)dimethylammonium chloride) offers a convenient way to prepare α-amino acid chlorides for peptide synthesis. Its use with N-trifluoracetyl (TFA)-protected isoleucine and allo-isoleucine is described. The 1H-NMR of the α-proton signal offers a convenient way to monitor the chirality retention in the acid chloride forming reaction and subsequent Friedel-Crafts acylation of arenes which result in α-amino acid aryl-ketone with no loss of chirality.
中文翻译:

用弗尔斯迈尔试剂进行弗里德尔-克拉夫特酰化反应的TFA保护的α-氨基酸氯化物的合成
α-氨基酸氯化物是酰胺(肽)形成的反应性偶联剂。Vilsmeier试剂(氯化(氯亚甲基)二甲基铵)提供了一种方便的方法来制备用于肽合成的α-氨基酸氯化物。描述了其与N-三氟乙酰基(TFA)保护的异亮氨酸和同种异亮氨酸一起使用。α-质子信号的1H-NMR提供了一种方便的方法来监控手性在酰基氯形成反应中的保留以及随后的芳烃的Friedel-Crafts酰化反应,这导致α-氨基酸芳基酮的存在而没有手性的损失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号