当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The catalytic mechanism of aromatic nitration by cytochrome P450 TxtE: Involvement of a ferric-peroxynitrite intermediate
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-19 , DOI: 10.1021/jacs.0c05070 Savvas Louka 1, 2 , Sarah M Barry 3 , Derren J Heyes 1, 4 , M Qadri E Mubarak 1, 2 , Hafiz Saqib Ali 1, 4 , Lona M Alkhalaf 3 , Andrew W Munro 1, 4 , Nigel S Scrutton 1, 4 , Gregory L Challis 3, 5, 6 , Sam P de Visser 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-19 , DOI: 10.1021/jacs.0c05070 Savvas Louka 1, 2 , Sarah M Barry 3 , Derren J Heyes 1, 4 , M Qadri E Mubarak 1, 2 , Hafiz Saqib Ali 1, 4 , Lona M Alkhalaf 3 , Andrew W Munro 1, 4 , Nigel S Scrutton 1, 4 , Gregory L Challis 3, 5, 6 , Sam P de Visser 1, 2
Affiliation
|
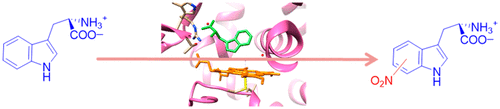
The cytochromes P450 are heme-dependent enzymes that catalyze many vital reaction processes in the human body related to biodegradation and biosynthesis. They typically act as mono-oxygenases; however, the recently discovered P450 subfamily TxtE utilizes O2 and NO to nitrate aromatic substrates such as L-tryptophan. A direct and selective aromatic nitration reaction may be useful in biotechnology for the synthesis of drugs or small molecules. Details of the catalytic mechanism are unknown, and it has been suggested that the reaction should proceed through either an iron(III)-superoxo or an iron(II)-nitrosyl intermediate. To resolve this controversy, we used stopped-flow kinetics to provide evidence for a catalytic cycle where dioxygen binds prior to NO to generate an active iron(III)-peroxynitrite species that is able to nitrate l-Trp efficiently. We show that the rate of binding of O2 is faster than that of NO and also leads to l-Trp nitration, while little evidence of product formation is observed from the iron(II)-nitrosyl complex. To support the experimental studies, we performed density functional theory studies on large active site cluster models. The studies suggest a mechanism involving an iron(III)-peroxynitrite that splits homolytically to form an iron(IV)-oxo heme (Compound II) and a free NO2 radical via a small free energy of activation. The latter activates the substrate on the aromatic ring, while compound II picks up the ipso-hydrogen to form the product. The calculations give small reaction barriers for most steps in the catalytic cycle and, therefore, predict fast product formation from the iron(III)-peroxynitrite complex. These findings provide the first detailed insight into the mechanism of nitration by a member of the TxtE subfamily and highlight how the enzyme facilitates this novel reaction chemistry.
中文翻译:

细胞色素 P450 TxtE 芳族硝化的催化机制:涉及过氧化亚硝酸铁中间体
细胞色素 P450 是血红素依赖性酶,可催化人体中与生物降解和生物合成相关的许多重要反应过程。它们通常充当单加氧酶;然而,最近发现的 P450 亚家族 TxtE 利用 O2 和 NO 来硝化芳香底物,例如 L-色氨酸。直接和选择性的芳族硝化反应可用于生物技术合成药物或小分子。催化机制的细节尚不清楚,有人建议该反应应该通过铁 (III)-超氧或铁 (II)-亚硝酰基中间体进行。为了解决这一争议,我们使用停流动力学为催化循环提供证据,其中分子氧在 NO 之前结合以生成能够有效硝化 l-Trp 的活性铁 (III)-过氧亚硝酸盐物种。我们表明 O2 的结合速率比 NO 的结合速率快,并且还导致 l-Trp 硝化,而从铁 (II)-亚硝酰基络合物中几乎没有观察到产物形成的证据。为了支持实验研究,我们对大型活动站点集群模型进行了密度泛函理论研究。这些研究提出了一种涉及铁 (III)-过氧亚硝酸盐的机制,它通过小的活化自由能均裂形成铁 (IV)-氧代血红素(化合物 II)和游离的 NO2 自由基。后者激活芳环上的底物,而化合物 II 吸收同氢以形成产物。该计算为催化循环中的大多数步骤提供了小的反应障碍,因此预测了铁 (III)-过氧亚硝酸盐络合物的快速产物形成。
更新日期:2020-08-19
中文翻译:

细胞色素 P450 TxtE 芳族硝化的催化机制:涉及过氧化亚硝酸铁中间体
细胞色素 P450 是血红素依赖性酶,可催化人体中与生物降解和生物合成相关的许多重要反应过程。它们通常充当单加氧酶;然而,最近发现的 P450 亚家族 TxtE 利用 O2 和 NO 来硝化芳香底物,例如 L-色氨酸。直接和选择性的芳族硝化反应可用于生物技术合成药物或小分子。催化机制的细节尚不清楚,有人建议该反应应该通过铁 (III)-超氧或铁 (II)-亚硝酰基中间体进行。为了解决这一争议,我们使用停流动力学为催化循环提供证据,其中分子氧在 NO 之前结合以生成能够有效硝化 l-Trp 的活性铁 (III)-过氧亚硝酸盐物种。我们表明 O2 的结合速率比 NO 的结合速率快,并且还导致 l-Trp 硝化,而从铁 (II)-亚硝酰基络合物中几乎没有观察到产物形成的证据。为了支持实验研究,我们对大型活动站点集群模型进行了密度泛函理论研究。这些研究提出了一种涉及铁 (III)-过氧亚硝酸盐的机制,它通过小的活化自由能均裂形成铁 (IV)-氧代血红素(化合物 II)和游离的 NO2 自由基。后者激活芳环上的底物,而化合物 II 吸收同氢以形成产物。该计算为催化循环中的大多数步骤提供了小的反应障碍,因此预测了铁 (III)-过氧亚硝酸盐络合物的快速产物形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号