当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Observation of Photoinduced Higher Oxidation States at a Semiconductor/Electrocatalyst Junction
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-18 , DOI: 10.1021/acscatal.0c02789 Francesco Malara 1 , Martina Fracchia 2 , Hana Kmentová 3 , Rinaldo Psaro 1 , Alberto Vertova 4 , Danilo Oliveira de Souza 5 , Giuliana Aquilanti 5 , Luca Olivi 5 , Paolo Ghigna 2, 6 , Alessandro Minguzzi 4, 6 , Alberto Naldoni 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-18 , DOI: 10.1021/acscatal.0c02789 Francesco Malara 1 , Martina Fracchia 2 , Hana Kmentová 3 , Rinaldo Psaro 1 , Alberto Vertova 4 , Danilo Oliveira de Souza 5 , Giuliana Aquilanti 5 , Luca Olivi 5 , Paolo Ghigna 2, 6 , Alessandro Minguzzi 4, 6 , Alberto Naldoni 3
Affiliation
|
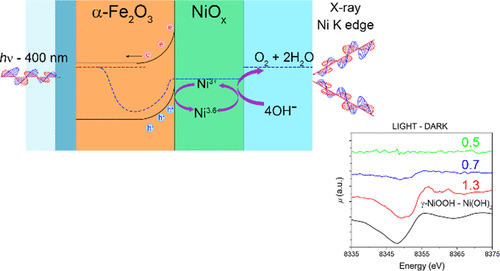
Photoelectrochemical (PEC) water splitting devices using semiconductors and electrocatalysts rely on heterogeneous interfaces that drive charge separation, thus determining potential gradients that dictate the reaction efficiency. The PEC potential of the electrocatalyst depends on the chemical oxidation state of forming elements, which may strongly vary under the photoinduced charge flow. However, element-sensitive, real-time measurements of the oxidation state of the electrocatalyst are not generally possible using conventional X-ray absorption techniques. Here, we show that fixed-energy X-ray absorption voltammetry and chronoamperometry, which measure the X-ray absorption coefficient variations along with photocurrent, can follow in real time the redox kinetics of electrocatalysts. To demonstrate the validity, we investigate hematite (α-Fe2O3) photoanodes covered with a nickel hydroxide electrocatalyst and show that it is fully oxidized by photogenerated holes to nickel oxyhydroxide with Ni reaching a higher oxidation state (NiIV) than that observed under electrocatalytic oxygen evolution in dark conditions. Highly oxidized Ni results from charge accumulation in the overlayer and can be observed only in the case of thick layers (with low PEC performance). On the other hand, the average oxidation state of Ni reaches lower values, under operative conditions, for very thin layers, resulting in high PEC activity. We complete our study by presenting PEC activity and impedance spectroscopy analysis using different thicknesses of the electrocatalyst, thus giving a detailed picture of the multiple and complex charge transfer processes occurring at a semiconductor/electrocatalyst junction.
中文翻译:

在半导体/电催化剂连接处直接观察光诱导的较高氧化态
使用半导体和电催化剂的光电化学(PEC)水分解装置依赖于驱动电荷分离的异质界面,从而确定了决定反应效率的电势梯度。电催化剂的PEC电位取决于形成元素的化学氧化态,在光诱导的电荷流下,该化学氧化态可能会发生很大变化。但是,使用常规的X射线吸收技术通常不可能对电催化剂的氧化态进行元素敏感的实时测量。在这里,我们表明,固定能量X射线吸收伏安法和计时电流法可以测量X射线吸收系数随光电流的变化,可以实时跟踪电催化剂的氧化还原动力学。为了证明其有效性,我们研究了赤铁矿(α-Fe2 O 3)覆盖有氢氧化镍电催化剂的光阳极,表明它被光生空穴完全氧化为羟基氧化镍,而Ni达到较高的氧化态(Ni IV)比在黑暗条件下在电催化氧气放出下观察到的 Ni的高度氧化是由于电荷在表层中的积累所致,并且仅在厚层(PEC性能较低)的情况下才能观察到。另一方面,对于非常薄的层,在操作条件下,Ni的平均氧化态达到较低的值,从而导致较高的PEC活性。我们通过介绍使用不同厚度的电催化剂的PEC活性和阻抗谱分析来完成我们的研究,从而详细描述发生在半导体/电催化剂结处的多种和复杂的电荷转移过程。
更新日期:2020-09-20
中文翻译:

在半导体/电催化剂连接处直接观察光诱导的较高氧化态
使用半导体和电催化剂的光电化学(PEC)水分解装置依赖于驱动电荷分离的异质界面,从而确定了决定反应效率的电势梯度。电催化剂的PEC电位取决于形成元素的化学氧化态,在光诱导的电荷流下,该化学氧化态可能会发生很大变化。但是,使用常规的X射线吸收技术通常不可能对电催化剂的氧化态进行元素敏感的实时测量。在这里,我们表明,固定能量X射线吸收伏安法和计时电流法可以测量X射线吸收系数随光电流的变化,可以实时跟踪电催化剂的氧化还原动力学。为了证明其有效性,我们研究了赤铁矿(α-Fe2 O 3)覆盖有氢氧化镍电催化剂的光阳极,表明它被光生空穴完全氧化为羟基氧化镍,而Ni达到较高的氧化态(Ni IV)比在黑暗条件下在电催化氧气放出下观察到的 Ni的高度氧化是由于电荷在表层中的积累所致,并且仅在厚层(PEC性能较低)的情况下才能观察到。另一方面,对于非常薄的层,在操作条件下,Ni的平均氧化态达到较低的值,从而导致较高的PEC活性。我们通过介绍使用不同厚度的电催化剂的PEC活性和阻抗谱分析来完成我们的研究,从而详细描述发生在半导体/电催化剂结处的多种和复杂的电荷转移过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号