当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IbpB-bound substrate release in living cells as revealed by unnatural amino acid-mediated photo-crosslinking.
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-08-19 , DOI: 10.1002/2211-5463.12957 Xiaodong Shi 1 , Anastasia N Ezemaduka 2
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-08-19 , DOI: 10.1002/2211-5463.12957 Xiaodong Shi 1 , Anastasia N Ezemaduka 2
Affiliation
|
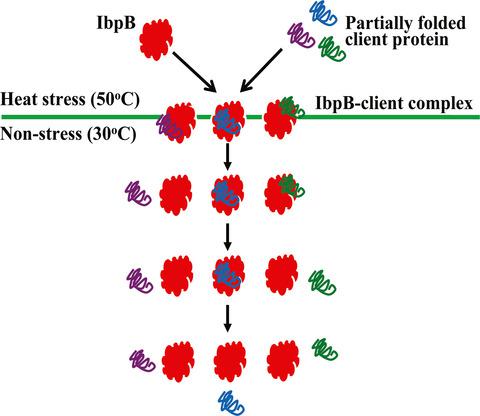
Small heat shock proteins (sHSPs) are known to bind non‐native substrates and prevent irreversible aggregation in an ATP‐independent manner. However, the dynamic interaction between sHSPs and their substrates in vivo is less studied. Here, by utilizing a genetically incorporated crosslinker, we characterized the interaction between sHSP IbpB and its endogenous substrates in living cells. Through photo‐crosslinking analysis of five Bpa variants of IbpB, we found that the substrate binding of IbpB in living cells is reversible upon short‐time exposure at 50 °C. Our data provide in vivo evidence that IbpB engages in dynamic substrate release under nonstress conditions and suggest that photo‐crosslinking may be a suitable method for investigating dynamic interaction between molecular chaperones and their substrates in living cells.
中文翻译:

非天然氨基酸介导的光交联揭示了活细胞中 IbpB 结合底物的释放。
已知小热休克蛋白 (sHSP) 可以结合非天然底物并以不依赖 ATP 的方式防止不可逆聚集。然而,对 sHSPs 与其底物在体内的动态相互作用的研究较少。在这里,通过利用基因结合的交联剂,我们表征了 sHSP IbpB 与其在活细胞中的内源性底物之间的相互作用。通过对 IbpB 的五种 Bpa 变体的光交联分析,我们发现 IbpB 在活细胞中的底物结合在 50°C 短时间暴露后是可逆的。我们的数据提供体内证据表明 IbpB 在非应激条件下参与动态底物释放,并表明光交联可能是研究活细胞中分子伴侣与其底物之间动态相互作用的合适方法。
更新日期:2020-10-02
中文翻译:

非天然氨基酸介导的光交联揭示了活细胞中 IbpB 结合底物的释放。
已知小热休克蛋白 (sHSP) 可以结合非天然底物并以不依赖 ATP 的方式防止不可逆聚集。然而,对 sHSPs 与其底物在体内的动态相互作用的研究较少。在这里,通过利用基因结合的交联剂,我们表征了 sHSP IbpB 与其在活细胞中的内源性底物之间的相互作用。通过对 IbpB 的五种 Bpa 变体的光交联分析,我们发现 IbpB 在活细胞中的底物结合在 50°C 短时间暴露后是可逆的。我们的数据提供体内证据表明 IbpB 在非应激条件下参与动态底物释放,并表明光交联可能是研究活细胞中分子伴侣与其底物之间动态相互作用的合适方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号