当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Corey Lactone and Latanoprost
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-08-19 , DOI: 10.1002/ejoc.202001063 Nariyoshi Umekubo 1 , Yujiro Hayashi 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-08-19 , DOI: 10.1002/ejoc.202001063 Nariyoshi Umekubo 1 , Yujiro Hayashi 1
Affiliation
|
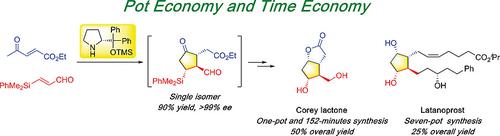
Corey lactone and latanoprost were synthesized in nearly enantiomeric pure forms following the concepts of pot and time economy using diphenylprolinol silyl ether mediated formal [3+2] cycloaddition reaction as key step. Corey lactone was synthesized in a one‐pot reaction within 152‐minutes in a total yield of 50 %, while latanoprost was synthesized through a seven‐pot synthesis with five purifications in a total yield of 25 %.

中文翻译:

科里内酯和拉坦前列素的不对称合成
按照二元脯氨醇甲硅烷基醚介导的正式[3 + 2]环加成反应为关键步骤,按照节省时间和时间的概念,以几乎对映体的纯形式合成了Corey内酯和latanoprost。Corey内酯是在152分钟内通过单锅反应合成的,总收率为50%,而拉坦前列素是通过7锅合成法经五次纯化合成的,总收率为25%。

更新日期:2020-10-17

中文翻译:

科里内酯和拉坦前列素的不对称合成
按照二元脯氨醇甲硅烷基醚介导的正式[3 + 2]环加成反应为关键步骤,按照节省时间和时间的概念,以几乎对映体的纯形式合成了Corey内酯和latanoprost。Corey内酯是在152分钟内通过单锅反应合成的,总收率为50%,而拉坦前列素是通过7锅合成法经五次纯化合成的,总收率为25%。












































 京公网安备 11010802027423号
京公网安备 11010802027423号