Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-19 , DOI: 10.1016/j.tet.2020.131472 Hirotaka Sasaki , Takashi Hosoya , Naoki Egami , Hina Ishimura , Akane Katori , Hisashi Miyafuji , Kouji Kuramochi , Ayumi Imayoshi , Kazunori Tsubaki
|
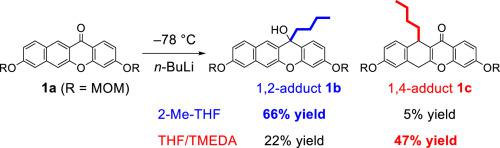
3,8-Bis(methoxymethoxy)benzo[b]xanthene-12-one (1a) was reacted with n-butyllithium at −78 °C to afford the expected 1,2-adduct 1b and also the 1,4-adduct 1c. Using 2-Me-THF as the solvent afforded predominantly the 1,2-adduct 1b in 66% yield and the 1,4-adduct 1c in 5% yield. In contrast, in THF, the 1,4-adduct 1c was obtained as the major product in 47% yield and the 1,2-adduct 1b was obtained in 22% yield. As a result of many experiments, it was found that these peculiar regioselectivity is due to a variety of factors such as the kind of solvents, the organometallic species, and the reaction substrates. Among them, the difference in regioselectivity between THF and 2-Me-THF was explained using theoretical calculations.
中文翻译:

苯并吨蒽酮衍生物的亲核加成反应与芳香性下降
3,8-双(甲氧基甲氧基)苯并[ b ] x吨-12-(1a)与正丁基锂在-78°C下反应,得到预期的1,2-加合物1b和1,4-加合物1c。用2-Me-THF作为溶剂,主要得到产率为66%的1,2-加合物1b和产率为5%的1,4-加合物1c。相反,在THF中,以1,4-加合物1c为主要产物,产率为47%,而1,2-加合物1b为主要产物。获得了22%的收率。作为许多实验的结果,发现这些特殊的区域选择性是由于多种因素引起的,例如溶剂的种类,有机金属种类和反应底物。其中,使用理论计算解释了THF和2-Me-THF之间的区域选择性的差异。









































 京公网安备 11010802027423号
京公网安备 11010802027423号