当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Radical-Triggered Reaction Mechanism of the Green-to-Red Photoconversion of EosFP.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.jpcb.0c04587 Clyde Fare 1, 2 , Letong Yuan 1 , Violeta Cordon-Preciado 1 , Jasper J Michels 3 , Michael J Bearpark 2 , Peter Rich 4 , Jasper J van Thor 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.jpcb.0c04587 Clyde Fare 1, 2 , Letong Yuan 1 , Violeta Cordon-Preciado 1 , Jasper J Michels 3 , Michael J Bearpark 2 , Peter Rich 4 , Jasper J van Thor 1
Affiliation
|
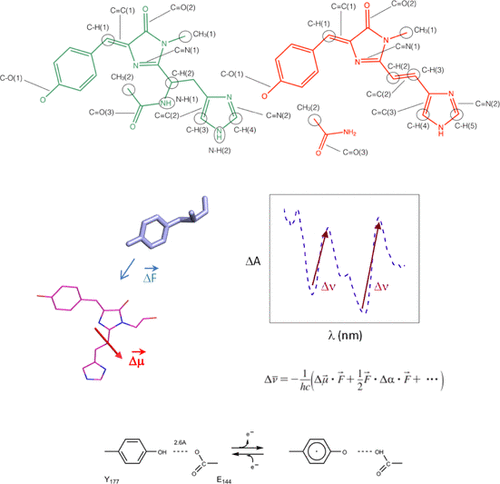
Reaction intermediates in the green-to-red photoconversion of the photochromic fluorescent protein EosFP have been observed using high-intensity continuous blue illumination. An intermediate was identified through light-induced accumulation that continues to convert the green form in subsequent darkness, putatively containing a tyrosyl radical, albeit with anomalously shifted features in both the electronic and FTIR spectra. Lowering the pH to 5.5 significantly delays the decay of this tyrosyl intermediate, which is accompanied by Stark-shifted features in the electronic spectra of reactants and products. Vibrational mode assignments for the high-frequency and fingerprint FTIR spectral regions of the reaction intermediates support a proposed sequence of events where the newly formed Cα═Cβ ethylenic bond precedes modifications on the His-62 imidazole ring and confirms a C═O(NH2) product group on Phe-61. We propose a reaction mechanism that involves tyrosyl generation via singlet excited-state-mediated oxidation which subsequently triggers the covalent reactions by oxidation of the green chromophore.
中文翻译:

EosFP的绿色到红色光电转换的自由基触发反应机理。
使用高强度连续蓝色照明可以观察到光致变色荧光蛋白EosFP从绿色到红色的光转换过程中的反应中间体。通过光诱导的积累鉴定出中间体,该中间体在随后的黑暗中继续转化为绿色形式,尽管其中电子和FTIR光谱均具有异常转移的特征,但仍推测含有酪氨酰自由基。将pH值降低至5.5会大大延迟该酪氨酰中间体的衰变,并伴随着反应物和产物电子光谱中的Stark位移特征。用于高频率和反应中间体的指纹FTIR光谱区域的振动模式分配支持其中新形成的事件的建议的序列C α = C β烯键在His-62咪唑环上进行修饰之前,并确认Phe-61上的C═O(NH 2)产物基团。我们提出了一种反应机制,该机制涉及通过单重态激发态介导的氧化产生酪氨酰,其随后通过绿色生色团的氧化触发共价反应。
更新日期:2020-09-10
中文翻译:

EosFP的绿色到红色光电转换的自由基触发反应机理。
使用高强度连续蓝色照明可以观察到光致变色荧光蛋白EosFP从绿色到红色的光转换过程中的反应中间体。通过光诱导的积累鉴定出中间体,该中间体在随后的黑暗中继续转化为绿色形式,尽管其中电子和FTIR光谱均具有异常转移的特征,但仍推测含有酪氨酰自由基。将pH值降低至5.5会大大延迟该酪氨酰中间体的衰变,并伴随着反应物和产物电子光谱中的Stark位移特征。用于高频率和反应中间体的指纹FTIR光谱区域的振动模式分配支持其中新形成的事件的建议的序列C α = C β烯键在His-62咪唑环上进行修饰之前,并确认Phe-61上的C═O(NH 2)产物基团。我们提出了一种反应机制,该机制涉及通过单重态激发态介导的氧化产生酪氨酰,其随后通过绿色生色团的氧化触发共价反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号