当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of Acid-Promoted N2 and N2O Generation from NH2NO and NH2NO2.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.jpca.0c06417 Tongheng Chen , Zhengyi Wan , Tarek Trabelsi , Chongqin Zhu , Joseph S. Francisco
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.jpca.0c06417 Tongheng Chen , Zhengyi Wan , Tarek Trabelsi , Chongqin Zhu , Joseph S. Francisco
|
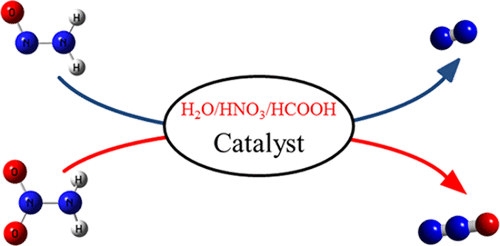
The effects of water, formic acid, and nitric acid on N2 and N2O generation from NH2NO and NH2NO2 are studied using high-level quantum-chemical calculations. It is shown that the reaction barriers from isolated NH2NO and NH2NO2 are 35.5 and 38.1 kcal/mol, respectively. When the NH2NO and NH2NO2 reactions are examined in the presence of water, formic acid, or nitric acid, the energy barriers are different. The mechanisms of these reactions are revealed through reaction pathway calculations. The isomerization of intermediate HNNOH and HNNOOH molecules, which follows a group rotation mechanism, plays a key role in reactions of isolated NH2NO and NH2NO2. However, the presence of water, formic acid, or nitric acid changes the isomerization mechanism substantially. HNNOH or HNNOOH and the catalyzing molecule form a doubly hydrogen-bonded prereactive complex, which, in turn, facilities hydrogen atom migration (this is denoted as the hydrogen atom migration mechanism). This study demonstrates the feasibility of N2 or N2O generation from NH2NO and NH2NO2 in the presence of water, formic acid, or nitric acid.
中文翻译:

由NH2NO和NH2NO2生成酸促进N2和N2O生成的机理。
使用高级量子化学计算研究了水,甲酸和硝酸对NH 2 NO和NH 2 NO 2生成N 2和N 2 O的影响。结果表明,分离出的NH 2 NO和NH 2 NO 2的反应势垒分别为35.5 kcal / mol和38.1 kcal / mol。当NH 2 NO和NH 2 NO 2在水,甲酸或硝酸的存在下检查反应,能量垒不同。这些反应的机理通过反应途径的计算得以揭示。遵循基团旋转机制的中间HNNOH和HNNOOH分子的异构化在分离的NH 2 NO和NH 2 NO 2的反应中起关键作用。然而,水,甲酸或硝酸的存在大大改变了异构化机理。HNNOH或HNNOOH与催化分子形成双氢键预反应络合物,进而促进氢原子迁移(这称为氢原子迁移机理)。这项研究证明了N 2或N的可行性2选自NH阿代2 NO和NH 2 NO 2中的水,甲酸,或硝酸的存在。
更新日期:2020-09-18
中文翻译:

由NH2NO和NH2NO2生成酸促进N2和N2O生成的机理。
使用高级量子化学计算研究了水,甲酸和硝酸对NH 2 NO和NH 2 NO 2生成N 2和N 2 O的影响。结果表明,分离出的NH 2 NO和NH 2 NO 2的反应势垒分别为35.5 kcal / mol和38.1 kcal / mol。当NH 2 NO和NH 2 NO 2在水,甲酸或硝酸的存在下检查反应,能量垒不同。这些反应的机理通过反应途径的计算得以揭示。遵循基团旋转机制的中间HNNOH和HNNOOH分子的异构化在分离的NH 2 NO和NH 2 NO 2的反应中起关键作用。然而,水,甲酸或硝酸的存在大大改变了异构化机理。HNNOH或HNNOOH与催化分子形成双氢键预反应络合物,进而促进氢原子迁移(这称为氢原子迁移机理)。这项研究证明了N 2或N的可行性2选自NH阿代2 NO和NH 2 NO 2中的水,甲酸,或硝酸的存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号