当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Upconverting Nanocarriers Enable Triggered Microtubule Inhibition and Concurrent Ferroptosis Induction for Selective Treatment of Triple-Negative Breast Cancer.
Nano Letters ( IF 9.6 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.nanolett.0c00502 Jundong Zhu 1 , Peipei Dai 1 , Fang Liu 1 , Yao Li 1 , Yan Qin 1 , Qian Yang 1 , Ran Tian 2 , Aiping Fan 1 , Simone de Fatima Medeiros 3 , Zheng Wang 1 , Yanjun Zhao 1
Nano Letters ( IF 9.6 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.nanolett.0c00502 Jundong Zhu 1 , Peipei Dai 1 , Fang Liu 1 , Yao Li 1 , Yan Qin 1 , Qian Yang 1 , Ran Tian 2 , Aiping Fan 1 , Simone de Fatima Medeiros 3 , Zheng Wang 1 , Yanjun Zhao 1
Affiliation
|
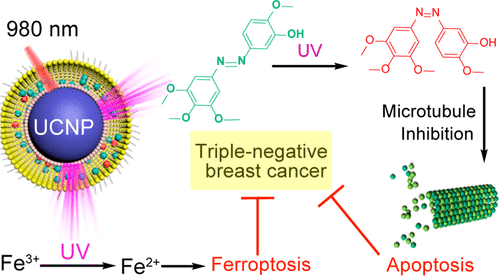
Despite the resistance of triple-negative breast cancer (TNBC) to targeted hormone therapy, the discovery of azobenzene combretastatin A4 (Azo-CA4) provides therapeutic opportunities for TNBC. Here, Azo-CA4 was loaded in upconverting nanocarriers that could convert near-infrared (NIR) light to UV light to activate Azo-CA4. Upon irradiation, Azo-CA4-loaded nanocarriers significantly reduced the viability of TNBC cells via both apoptosis and ferroptosis. The former was induced by photoisomerization of Azo-CA4, accompanied by microtubule breakdown and cell cycle arrest at G2/M phase. The latter was caused by the UV light-induced reduction of Fe3+ to Fe2+ that facilitates the peroxidation of tailored lipids. The cooperation between apoptosis and ferroptosis in eliminating TNBC was demonstrated in a xenograft mice model in terms of histological staining, tumor growth inhibition, and animal survival. Since the NIR light is only applied to the tumor site, the adverse effects of such triggered nanocarriers to the healthy organs are negligible.
中文翻译:

上转换纳米载体使触发的微管抑制和并发肥大病的诱导选择性治疗三阴性乳腺癌。
尽管三阴性乳腺癌(TNBC)对靶向激素治疗有抵抗力,但偶氮苯康他汀A4(Azo-CA4)的发现为TNBC提供了治疗机会。在这里,Azo-CA4被加载到可以将近红外(NIR)光转换为UV光以激活Azo-CA4的上转换纳米载体中。照射后,载有偶氮-CA4的纳米载体会通过凋亡和肥大作用显着降低TNBC细胞的活力。前者是由Azo-CA4的光异构化诱导的,伴随着微管分解和细胞周期阻滞在G 2 / M期。后者是由紫外线引起的Fe 3+还原为Fe 2+引起的促进特制脂质的过氧化。在异种移植小鼠模型中,从组织学染色,肿瘤生长抑制和动物存活方面证明了凋亡和铁肥作用在消除TNBC方面的合作。由于仅将NIR光施加到肿瘤部位,所以这种触发的纳米载体对健康器官的不利影响可以忽略不计。
更新日期:2020-09-10
中文翻译:

上转换纳米载体使触发的微管抑制和并发肥大病的诱导选择性治疗三阴性乳腺癌。
尽管三阴性乳腺癌(TNBC)对靶向激素治疗有抵抗力,但偶氮苯康他汀A4(Azo-CA4)的发现为TNBC提供了治疗机会。在这里,Azo-CA4被加载到可以将近红外(NIR)光转换为UV光以激活Azo-CA4的上转换纳米载体中。照射后,载有偶氮-CA4的纳米载体会通过凋亡和肥大作用显着降低TNBC细胞的活力。前者是由Azo-CA4的光异构化诱导的,伴随着微管分解和细胞周期阻滞在G 2 / M期。后者是由紫外线引起的Fe 3+还原为Fe 2+引起的促进特制脂质的过氧化。在异种移植小鼠模型中,从组织学染色,肿瘤生长抑制和动物存活方面证明了凋亡和铁肥作用在消除TNBC方面的合作。由于仅将NIR光施加到肿瘤部位,所以这种触发的纳米载体对健康器官的不利影响可以忽略不计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号