当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-Acting Efavirenz and HIV-1 Fusion Inhibitor Peptide Co-loaded Polymer-Lipid Hybrid Nanoparticles: Statistical Optimization, Cellular Uptake, and In Vivo Biodistribution.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.molpharmaceut.0c00773 Dhanashree H Surve 1 , Yugandhara B Jirwankar 2 , Vikas D Dighe 2 , Anil B Jindal 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-18 , DOI: 10.1021/acs.molpharmaceut.0c00773 Dhanashree H Surve 1 , Yugandhara B Jirwankar 2 , Vikas D Dighe 2 , Anil B Jindal 1
Affiliation
|
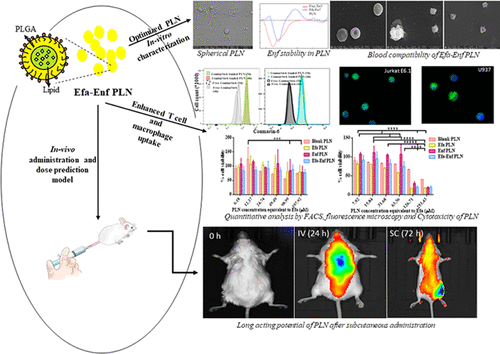
The objective of the present study was to develop long-acting efavirenz (Efa)–enfuvirtide (Enf) Co-loaded polymer–lipid hybrid nanoparticles (PLN) with improved intracellular delivery to target T-cells and macrophage cells located in multiple human immunodeficiency virus sanctuaries. The Box–Behnken design was utilized to optimize three high-risk factors, namely, Efa amount, sonication time for primary emulsion, and sonication time for aqueous nanodispersion obtained from preliminary studies. Lyophilized Efa–Enf Co-loaded PLN using trehalose elicited spherical morphology, drug amorphization on incorporation, and absence of drug-excipient interaction. In vitro release studies revealed an sustained release of both the drugs from PLN with the differential release profile. Efa–Enf Co-loaded PLN exhibited low hemolytic, platelet and leukocyte aggregation as well as low cytotoxicity in Jurkat E6.1 T-cells and U937 macrophage cells. Circular dichroism spectra confirmed the presence of an α-helix form of Enf after encapsulation in PLN. Coumarin-6-loaded PLN exhibited enhanced cellular uptake in Jurkat E6.1 T-cells and U937 macrophage cells in comparison to free coumarin-6, as evidenced by fluorescence microscopy and flow cytometry. In vivo biodistribution studies after intravenous administration of near-infrared dye-loaded PLN (surrogate for Efa–Enf PLN) revealed non-uniform distribution within 2 h in the order of spleen ≥ liver > lymph node > thymus > lungs > female reproductive tract (FRT) > heart > kidneys > brain. However, subcutaneous administration caused non-uniform biodistribution after 3 days, eliciting a long-acting slow release from the injection site depot until day 5 in the infection-spread site (lymph nodes and FRT), reservoir sites (liver and spleen) and the difficult-to-access site (brain). Furthermore, it presents a vital illustration of the available tissue-specific drug concentration prediction from simulated surrogate PLN.
中文翻译:

长效依法韦仑和 HIV-1 融合抑制剂肽共载聚合物-脂质混合纳米颗粒:统计优化、细胞摄取和体内生物分布。
本研究的目的是开发长效依非韦伦 (Efa)-恩夫韦肽 (Enf) 共载聚合物-脂质杂化纳米粒子 (PLN),改善细胞内递送至位于多种人类免疫缺陷病毒中的靶向 T 细胞和巨噬细胞。圣所。Box-Behnken 设计用于优化三个高风险因素,即 Efa 量、初级乳液的超声处理时间和初步研究中获得的水性纳米分散体的超声处理时间。使用海藻糖的冻干 Efa-Enf 共载 PLN 引发球形形态、掺入时药物无定形化以及没有药物-赋形剂相互作用。体外释放研究显示两种药物从 PLN 中缓释,释放曲线不同。Efa-Enf 共载 PLN 在 Jurkat E6.1 T 细胞和 U937 巨噬细胞中表现出低溶血性、血小板和白细胞聚集以及低细胞毒性。圆二色光谱证实在 PLN 中封装后存在 α-螺旋形式的 Enf。与游离香豆素 6 相比,载有香豆素 6 的 PLN 在 Jurkat E6.1 T 细胞和 U937 巨噬细胞中表现出增强的细胞摄取,荧光显微镜和流式细胞术证明了这一点。体内静脉注射载有近红外染料的 PLN(Efa-Enf PLN 的替代品)后的生物分布研究显示,2 小时内分布不均匀,顺序为脾≥肝 > 淋巴结 > 胸腺 > 肺 > 女性生殖道 (FRT) > 心脏 > 肾脏 > 大脑。然而,皮下给药在 3 天后导致不均匀的生物分布,导致在感染扩散部位(淋巴结和 FRT)、储存部位(肝脏和脾脏)和第 5 天从注射部位贮库长效缓慢释放。难以访问的网站(大脑)。此外,它提供了模拟替代 PLN 中可用的组织特异性药物浓度预测的重要说明。
更新日期:2020-10-05
中文翻译:

长效依法韦仑和 HIV-1 融合抑制剂肽共载聚合物-脂质混合纳米颗粒:统计优化、细胞摄取和体内生物分布。
本研究的目的是开发长效依非韦伦 (Efa)-恩夫韦肽 (Enf) 共载聚合物-脂质杂化纳米粒子 (PLN),改善细胞内递送至位于多种人类免疫缺陷病毒中的靶向 T 细胞和巨噬细胞。圣所。Box-Behnken 设计用于优化三个高风险因素,即 Efa 量、初级乳液的超声处理时间和初步研究中获得的水性纳米分散体的超声处理时间。使用海藻糖的冻干 Efa-Enf 共载 PLN 引发球形形态、掺入时药物无定形化以及没有药物-赋形剂相互作用。体外释放研究显示两种药物从 PLN 中缓释,释放曲线不同。Efa-Enf 共载 PLN 在 Jurkat E6.1 T 细胞和 U937 巨噬细胞中表现出低溶血性、血小板和白细胞聚集以及低细胞毒性。圆二色光谱证实在 PLN 中封装后存在 α-螺旋形式的 Enf。与游离香豆素 6 相比,载有香豆素 6 的 PLN 在 Jurkat E6.1 T 细胞和 U937 巨噬细胞中表现出增强的细胞摄取,荧光显微镜和流式细胞术证明了这一点。体内静脉注射载有近红外染料的 PLN(Efa-Enf PLN 的替代品)后的生物分布研究显示,2 小时内分布不均匀,顺序为脾≥肝 > 淋巴结 > 胸腺 > 肺 > 女性生殖道 (FRT) > 心脏 > 肾脏 > 大脑。然而,皮下给药在 3 天后导致不均匀的生物分布,导致在感染扩散部位(淋巴结和 FRT)、储存部位(肝脏和脾脏)和第 5 天从注射部位贮库长效缓慢释放。难以访问的网站(大脑)。此外,它提供了模拟替代 PLN 中可用的组织特异性药物浓度预测的重要说明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号