当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Light-Driven Allosteric Control of GPCR Biological Activity
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-08-18 , DOI: 10.1021/acsptsci.0c00054 Maria Ricart-Ortega 1, 2 , Alice E Berizzi 2 , Vanessa Pereira 2 , Fanny Malhaire 2 , Juanlo Catena 1 , Joan Font 2 , Xavier Gómez-Santacana 2 , Lourdes Muñoz 1, 3 , Charleine Zussy 2 , Carmen Serra 1, 3 , Xavier Rovira 1 , Cyril Goudet 2 , Amadeu Llebaria 1, 3
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-08-18 , DOI: 10.1021/acsptsci.0c00054 Maria Ricart-Ortega 1, 2 , Alice E Berizzi 2 , Vanessa Pereira 2 , Fanny Malhaire 2 , Juanlo Catena 1 , Joan Font 2 , Xavier Gómez-Santacana 2 , Lourdes Muñoz 1, 3 , Charleine Zussy 2 , Carmen Serra 1, 3 , Xavier Rovira 1 , Cyril Goudet 2 , Amadeu Llebaria 1, 3
Affiliation
|
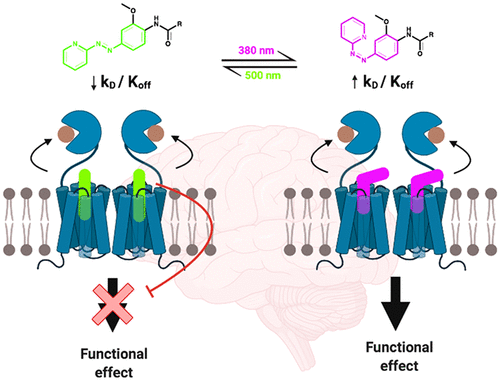
G protein-coupled receptors (GPCR), including the metabotrobic glutamate 5 receptor (mGlu5), are important therapeutic targets and the development of allosteric ligands for targeting GPCRs has become a desirable approach toward modulating receptor activity. Traditional pharmacological approaches toward modulating GPCR activity are still limited since precise spatiotemporal control of a ligand is lost as soon as it is administered. Photopharmacology proposes the use of photoswitchable ligands to overcome this limitation, since their activity can be reversibly controlled by light with high precision. As this is still a growing field, our understanding of the molecular mechanisms underlying the light-induced changes of different photoswitchable ligand pharmacology is suboptimal. For this reason, we have studied the mechanisms of action of alloswitch-1 and MCS0331; two freely diffusible, mGlu5 phenylazopyridine photoswitchable negative allosteric modulators. We combined photochemical, cell-based, and in vivo photopharmacological approaches to investigate the effects of trans–cis azobenzene photoisomerization on the functional activity and binding ability of these ligands to the mGlu5 allosteric pocket. From these results, we conclude that photoisomerization can take place inside and outside the ligand binding pocket, and this leads to a reversible loss in affinity, in part, due to changes in dissociation rates from the receptor. Ligand activity for both photoswitchable ligands deviates from high-affinity mGlu5 negative allosteric modulation (in the trans configuration) to reduced affinity for the mGlu5 in their cis configuration. Importantly, this mechanism translates to dynamic and reversible control over pain following local injection and illumination of negative allosteric modulators into a brain region implicated in pain control.
中文翻译:

对 GPCR 生物活性的光驱动变构控制的机械洞察
G 蛋白偶联受体 (GPCR),包括代谢型谷氨酸 5 受体 (mGlu 5),是重要的治疗靶点,开发用于靶向 GPCR 的变构配体已成为调节受体活性的理想方法。调节 GPCR 活性的传统药理学方法仍然有限,因为配体一经给药就失去了对配体的精确时空控制。光药理学建议使用光可切换配体来克服这一限制,因为它们的活性可以由光以高精度可逆地控制。由于这仍然是一个不断发展的领域,我们对不同光可开关配体药理学光诱导变化的分子机制的理解并不理想。为此,我们研究了alloswitch-1和MCS0331的作用机制;两个可自由扩散的 mGlu 5苯基偶氮吡啶光开关负变构调节剂。我们结合光化学、基于细胞和体内光药理学方法来研究反式-顺式偶氮苯光异构化对这些配体与 mGlu 5变构口袋的功能活性和结合能力的影响。从这些结果,我们得出结论,光异构化可以发生在配体结合口袋的内部和外部,这导致亲和力的可逆损失,部分原因是受体解离速率的变化。两种光开关配体的配体活性都偏离高亲和力 mGlu 5负变构调节(在反式中)配置)以降低其顺式配置中对 mGlu 5 的亲和力。重要的是,在将负变构调节剂局部注射和照射到与疼痛控制有关的大脑区域后,这种机制转化为对疼痛的动态和可逆控制。
更新日期:2020-10-11
中文翻译:

对 GPCR 生物活性的光驱动变构控制的机械洞察
G 蛋白偶联受体 (GPCR),包括代谢型谷氨酸 5 受体 (mGlu 5),是重要的治疗靶点,开发用于靶向 GPCR 的变构配体已成为调节受体活性的理想方法。调节 GPCR 活性的传统药理学方法仍然有限,因为配体一经给药就失去了对配体的精确时空控制。光药理学建议使用光可切换配体来克服这一限制,因为它们的活性可以由光以高精度可逆地控制。由于这仍然是一个不断发展的领域,我们对不同光可开关配体药理学光诱导变化的分子机制的理解并不理想。为此,我们研究了alloswitch-1和MCS0331的作用机制;两个可自由扩散的 mGlu 5苯基偶氮吡啶光开关负变构调节剂。我们结合光化学、基于细胞和体内光药理学方法来研究反式-顺式偶氮苯光异构化对这些配体与 mGlu 5变构口袋的功能活性和结合能力的影响。从这些结果,我们得出结论,光异构化可以发生在配体结合口袋的内部和外部,这导致亲和力的可逆损失,部分原因是受体解离速率的变化。两种光开关配体的配体活性都偏离高亲和力 mGlu 5负变构调节(在反式中)配置)以降低其顺式配置中对 mGlu 5 的亲和力。重要的是,在将负变构调节剂局部注射和照射到与疼痛控制有关的大脑区域后,这种机制转化为对疼痛的动态和可逆控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号