当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity-Controlling Factors in Carboxylate-Assisted C–H Activation under 4d and 3d Transition Metal Catalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-17 , DOI: 10.1021/acscatal.0c02808 Torben Rogge 1 , João C. A. Oliveira 1 , Rositha Kuniyil 1 , Lianrui Hu 1 , Lutz Ackermann 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-17 , DOI: 10.1021/acscatal.0c02808 Torben Rogge 1 , João C. A. Oliveira 1 , Rositha Kuniyil 1 , Lianrui Hu 1 , Lutz Ackermann 1
Affiliation
|
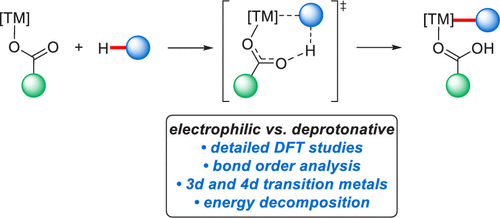
Detailed density functional theory calculations provide valuable insight into reactivity-controlling factors in transition metal-catalyzed C–H activation by carboxylate assistance. The chelation-assisted activation of a variety of arenes by 3d and 4d transition metal complexes was analyzed by means of bond order analysis through density functional theory (DFT) calculations as well as energy decomposition analysis through DLPNO–CCSD(T) calculations, thereby providing in-depth information on distinct electronic influences on the key C–H activation transition state and demonstrating a preferred activation through a base-assisted internal electrophilic substitution (BIES) rather than a concerted metalation-deprotonation (CMD) pathway.
中文翻译:

4d和3d过渡金属催化下羧酸盐辅助的C–H活化中的反应性控制因素
详细的密度泛函理论计算为过渡金属催化的羧酸盐辅助的C–H活化中的反应性控制因素提供了有价值的见解。通过键序分析(通过密度泛函理论(DFT)计算)以及能量分解分析(通过DLPNO–CCSD(T)计算),分析了3d和4d过渡金属配合物对多种芳烃的螯合辅助活化有关关键C–H活化转变状态的不同电子影响的深入信息,并通过碱辅助内部亲电取代(BIES)而非协同的金属化-去质子化(CMD)途径证明了优选的活化方式。
更新日期:2020-09-20
中文翻译:

4d和3d过渡金属催化下羧酸盐辅助的C–H活化中的反应性控制因素
详细的密度泛函理论计算为过渡金属催化的羧酸盐辅助的C–H活化中的反应性控制因素提供了有价值的见解。通过键序分析(通过密度泛函理论(DFT)计算)以及能量分解分析(通过DLPNO–CCSD(T)计算),分析了3d和4d过渡金属配合物对多种芳烃的螯合辅助活化有关关键C–H活化转变状态的不同电子影响的深入信息,并通过碱辅助内部亲电取代(BIES)而非协同的金属化-去质子化(CMD)途径证明了优选的活化方式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号