当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate specificities of inteins investigated by QuickDrop‐cassette mutagenesis
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-26 , DOI: 10.1002/1873-3468.13909 Jesper S Oeemig 1 , Hannes M Beyer 1 , A Sesilja Aranko 1 , Justus Mutanen 1 , Hideo Iwaï 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-26 , DOI: 10.1002/1873-3468.13909 Jesper S Oeemig 1 , Hannes M Beyer 1 , A Sesilja Aranko 1 , Justus Mutanen 1 , Hideo Iwaï 1
Affiliation
|
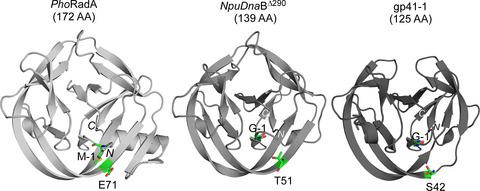
Inteins catalyze self‐excision from host precursor proteins while concomitantly ligating the flanking substrates (exteins) with a peptide bond. Noncatalytic extein residues near the splice junctions, such as the residues at the −1 and +2 positions, often strongly influence the protein‐splicing efficiency. The substrate specificities of inteins have not been studied for many inteins. We developed a convenient mutagenesis platform termed “QuickDrop”‐cassette mutagenesis for investigating the influences of 20 amino acid types at the −1 and +2 positions of different inteins. We elucidated 17 different profiles of the 20 amino acid dependencies across different inteins. The substrate specificities will accelerate our understanding of the structure–function relationship at the splicing junctions for broader applications of inteins in biotechnology and molecular biosciences.
中文翻译:

通过 QuickDrop-cassette 诱变研究内含肽的底物特异性
内含肽催化宿主前体蛋白的自我切除,同时用肽键连接侧翼底物(外显肽)。剪接点附近的非催化外显子残基,例如 -1 和 +2 位置的残基,通常会强烈影响蛋白质剪接效率。许多内含肽的底物特异性尚未研究过。我们开发了一个方便的诱变平台,称为“QuickDrop”-盒式诱变,用于研究不同内含肽 -1 和 +2 位置的 20 种氨基酸类型的影响。我们阐明了跨不同内含肽的 20 种氨基酸依赖性的 17 种不同谱。
更新日期:2020-09-26
中文翻译:

通过 QuickDrop-cassette 诱变研究内含肽的底物特异性
内含肽催化宿主前体蛋白的自我切除,同时用肽键连接侧翼底物(外显肽)。剪接点附近的非催化外显子残基,例如 -1 和 +2 位置的残基,通常会强烈影响蛋白质剪接效率。许多内含肽的底物特异性尚未研究过。我们开发了一个方便的诱变平台,称为“QuickDrop”-盒式诱变,用于研究不同内含肽 -1 和 +2 位置的 20 种氨基酸类型的影响。我们阐明了跨不同内含肽的 20 种氨基酸依赖性的 17 种不同谱。










































 京公网安备 11010802027423号
京公网安备 11010802027423号