当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Non-aqueous H3 PO4 Electrolyte Enables Stable Cycling of Proton Electrodes.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-17 , DOI: 10.1002/anie.202010554 Yunkai Xu 1 , Xianyong Wu 1 , Heng Jiang 1 , Longteng Tang 1 , Kenneth Y Koga 1 , Chong Fang 1 , Jun Lu 2 , Xiulei Ji 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-17 , DOI: 10.1002/anie.202010554 Yunkai Xu 1 , Xianyong Wu 1 , Heng Jiang 1 , Longteng Tang 1 , Kenneth Y Koga 1 , Chong Fang 1 , Jun Lu 2 , Xiulei Ji 1
Affiliation
|
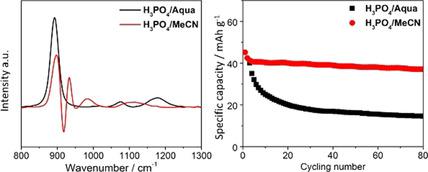
A non‐aqueous proton electrolyte is devised by dissolving H3PO4 into acetonitrile. The electrolyte exhibits unique vibrational signatures from stimulated Raman spectroscopy. Such an electrolyte exhibits unique characteristics compared to aqueous acidic electrolytes: 1) higher (de)protonation potential for a lower desolvation energy of protons, 2) better cycling stability by dissolution suppression, and 3) higher Coulombic efficiency owing to the lack of oxygen evolution reaction. Two non‐aqueous proton full cells exhibit better cycling stability, higher Coulombic efficiency, and less self‐discharge compared to the aqueous counterpart.
中文翻译:

非水H3 PO4电解质可实现质子电极的稳定循环。
通过将H 3 PO 4溶解在乙腈中,设计出一种非水质子电解质。电解质表现出受激拉曼光谱的独特振动特征。与水性酸性电解质相比,这种电解质表现出独特的特性:1)较高的(去质子化)势能以较低的质子脱溶剂能; 2)通过抑制溶解获得更好的循环稳定性;以及3)由于缺乏氧气逸出而产生的库仑效率更高反应。与水性同行相比,两个非水性质子充满细胞表现出更好的循环稳定性,更高的库仑效率和更少的自放电。
更新日期:2020-08-17
中文翻译:

非水H3 PO4电解质可实现质子电极的稳定循环。
通过将H 3 PO 4溶解在乙腈中,设计出一种非水质子电解质。电解质表现出受激拉曼光谱的独特振动特征。与水性酸性电解质相比,这种电解质表现出独特的特性:1)较高的(去质子化)势能以较低的质子脱溶剂能; 2)通过抑制溶解获得更好的循环稳定性;以及3)由于缺乏氧气逸出而产生的库仑效率更高反应。与水性同行相比,两个非水性质子充满细胞表现出更好的循环稳定性,更高的库仑效率和更少的自放电。











































 京公网安备 11010802027423号
京公网安备 11010802027423号