当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Chemotherapy of p53‐Mutated Cancer through Ubiquitination‐Dependent Proteasomal Degradation of Mutant p53 Proteins by Engineered ZnFe‐4 Nanoparticles
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-08-17 , DOI: 10.1002/adfm.202001994 Jieying Qian 1, 2 , Wenbin Zhang 1, 3 , Pengfei Wei 1 , Guangyu Yao 4 , Tianxiang Yi 1, 2 , Hao Zhang 1, 2 , He Ding 1, 3 , Xiaowan Huang 1, 2 , Meimei Wang 1, 2 , Yang Song 1, 5 , Suqin Zhong 1, 2 , Lijiao Yang 6 , Jinhao Gao 6 , Zijian Zhou 6 , Long‐ping Wen 1, 2, 7 , Yunjiao Zhang 1, 3, 5
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-08-17 , DOI: 10.1002/adfm.202001994 Jieying Qian 1, 2 , Wenbin Zhang 1, 3 , Pengfei Wei 1 , Guangyu Yao 4 , Tianxiang Yi 1, 2 , Hao Zhang 1, 2 , He Ding 1, 3 , Xiaowan Huang 1, 2 , Meimei Wang 1, 2 , Yang Song 1, 5 , Suqin Zhong 1, 2 , Lijiao Yang 6 , Jinhao Gao 6 , Zijian Zhou 6 , Long‐ping Wen 1, 2, 7 , Yunjiao Zhang 1, 3, 5
Affiliation
|
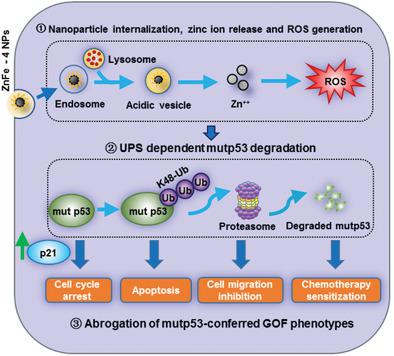
A significant percentage of human cancers harbor missense mutations in the TP53 gene and express highly stabilized mutant p53 protein (mutp53) with tumor‐promoting gain‐of‐function (GOF) properties. Inducing mutp53 degradation is a viable precision anti‐tumor therapeutic strategy. Based on the previously reported finding that a zinc‐curcumin compound induced mutp53 degradation, a series of ZnFe nanoparticles (ZnFe NPs) are synthesized and it is found that ZnFe‐4, with an Zn:Fe ratio of 1:2, exhibits outstanding mutp53‐degrading capability. ZnFe‐4 induced ubiquitination‐mediated proteasomal degradation of several different mutp53 species, but not the wild‐type p53 protein. Cellular internalization, intracellular Zn++ elevation and increased ROS are all necessary for ZnFe‐4‐induced mutp53 degradation. Degradation of mutp53 by ZnFe‐4, abrogated mutp53‐manifested GOF, leading to increased p21 expression, cell cycle arrest, reduced cell proliferation and cell migration, and cell demise. ZnFe‐4 also sensitized to cisplatin‐elicited killing in p53 S241F ES‐2 ovarian cancer cells, and dramatically improved the therapeutic efficacy of cisplatin in a subcutaneous ES‐2 tumor model. The potential clinical utility of ZnFe‐4 is further demonstrated in an orthotopically‐implanted p53 Y220C patient‐derived xenograft (PDX) breast cancer model. ZnFe‐4 is the first reported mutp53‐degrading nanomaterial, and further materials engineering may lead to the development of zinc‐based nanoparticles with minimal toxicity and maximized mutp53‐degrading capability.
中文翻译:

通过工程化的ZnFe-4纳米粒子通过泛素化依赖的蛋白酶体降解突变型p53蛋白来增强p53突变的癌症的化学治疗
很大比例的人类癌症在TP53基因中存在错义突变,并表达高度稳定的突变p53蛋白(mutp53),具有促进肿瘤的功能获得(GOF)特性。诱导mutp53降解是一种可行的精密抗肿瘤治疗策略。基于先前报道的锌-姜黄素化合物诱导的mutp53降解的发现,合成了一系列ZnFe纳米颗粒(ZnFe NPs),并且发现ZnFe比例为1:2的ZnFe-4表现出出色的mutp53。降解能力。ZnFe-4诱导了几种不同的mutp53物种的泛素介导的蛋白酶体降解,但未诱导野生型p53蛋白。细胞内在化,细胞内Zn ++升高和ROS升高都是ZnFe-4诱导的mutp53降解所必需的。ZnFe-4对mutp53的降解 废除mutp53表现的GOF,导致p21表达增加,细胞周期停滞,细胞增殖和细胞迁移减少以及细胞死亡。ZnFe-4还对顺铂引起的p53 S241F ES-2卵巢癌细胞的杀伤敏感,并显着提高了顺铂在皮下ES-2肿瘤模型中的治疗效果。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4还对顺铂引起的p53 S241F ES-2卵巢癌细胞的杀伤敏感,并显着提高了顺铂在皮下ES-2肿瘤模型中的治疗效果。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4还对顺铂引起的p53 S241F ES-2卵巢癌细胞的杀伤敏感,并显着提高了顺铂在皮下ES-2肿瘤模型中的治疗效果。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。
更新日期:2020-10-02
中文翻译:

通过工程化的ZnFe-4纳米粒子通过泛素化依赖的蛋白酶体降解突变型p53蛋白来增强p53突变的癌症的化学治疗
很大比例的人类癌症在TP53基因中存在错义突变,并表达高度稳定的突变p53蛋白(mutp53),具有促进肿瘤的功能获得(GOF)特性。诱导mutp53降解是一种可行的精密抗肿瘤治疗策略。基于先前报道的锌-姜黄素化合物诱导的mutp53降解的发现,合成了一系列ZnFe纳米颗粒(ZnFe NPs),并且发现ZnFe比例为1:2的ZnFe-4表现出出色的mutp53。降解能力。ZnFe-4诱导了几种不同的mutp53物种的泛素介导的蛋白酶体降解,但未诱导野生型p53蛋白。细胞内在化,细胞内Zn ++升高和ROS升高都是ZnFe-4诱导的mutp53降解所必需的。ZnFe-4对mutp53的降解 废除mutp53表现的GOF,导致p21表达增加,细胞周期停滞,细胞增殖和细胞迁移减少以及细胞死亡。ZnFe-4还对顺铂引起的p53 S241F ES-2卵巢癌细胞的杀伤敏感,并显着提高了顺铂在皮下ES-2肿瘤模型中的治疗效果。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4还对顺铂引起的p53 S241F ES-2卵巢癌细胞的杀伤敏感,并显着提高了顺铂在皮下ES-2肿瘤模型中的治疗效果。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4还对顺铂引起的p53 S241F ES-2卵巢癌细胞的杀伤敏感,并显着提高了顺铂在皮下ES-2肿瘤模型中的治疗效果。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。ZnFe-4的潜在临床实用性在原位植入的p53 Y220C患者异种移植(PDX)乳腺癌模型中得到了进一步证明。ZnFe-4是第一个报道的可降解mutp53的纳米材料,进一步的材料工程可能会导致锌基纳米颗粒的开发,其具有最小的毒性和最大的mutp53降解能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号