Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-18 , DOI: 10.1016/j.tetlet.2020.152378 Subhankar Ghosh , Rajat Ghosh , Shital K. Chattopadhyay
|
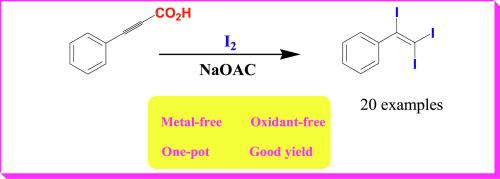
A metal- and oxidant-free facile synthesis of a range of 1,1,2-triiodostryrene derivatives has been developed which utilizes a simple decarboxylative triiodination of propiolic acids using molecular iodine and sodium acetate in a one-pot manner. Electron-withdrawing or donating substituents in the aryl rings display marginal influence on the course of the reaction. Mechanistic investigation reveals that the reaction proceeds via a mono-iodo alkyne derivative which subsequently adds an iodine molecule to provide the title compounds. On the other hand, β,β-diarylacrylic acids, under identical conditions undergo only decarboxylative mono-iodinaion to provide 1,1-diaryl-2-iodoalkenes, which do not undergo further iodination. The scope of the latter reaction was also examined.
中文翻译:

通过一锅丙酸的十烷氧基化碘化反应,无需氧化剂和添加剂即可轻松合成1,1,2-三碘代苯乙烯
已经开发了一系列无金属和无氧化剂的1,1,2-三碘代苯乙烯衍生物,该合成方法利用分子碘和乙酸钠以一锅法利用丙酸的简单脱羧三碘化。芳基环中的吸电子或给电子取代基对反应过程显示出很小的影响。机理研究表明,反应是通过单碘炔烃衍生物进行的,该衍生物随后加入碘分子以提供标题化合物。另一方面,在相同条件下,β,β-二芳基丙烯酸仅经历脱羧单碘化以提供1,1-二芳基-2-碘代烯烃,其不进行进一步的碘化。还检查了后者反应的范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号