Structure ( IF 4.4 ) Pub Date : 2020-08-18 , DOI: 10.1016/j.str.2020.07.016 Franziska Preuss 1 , Deep Chatterjee 1 , Sebastian Mathea 1 , Safal Shrestha 2 , Jonathan St-Germain 3 , Manipa Saha 3 , Natarajan Kannan 2 , Brian Raught 3 , Robert Rottapel 4 , Stefan Knapp 5
|
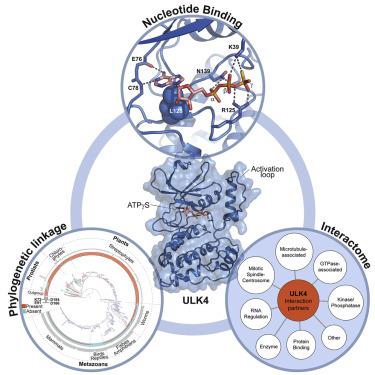
Unc-51-like kinase 4 (ULK4) is a pseudokinase that has been linked to the development of several diseases. Even though sequence motifs required for ATP binding in kinases are lacking, ULK4 still tightly binds ATP and the presence of the co-factor is required for structural stability of ULK4. Here, we present a high-resolution structure of a ULK4-ATPγS complex revealing a highly unusual ATP binding mode in which the lack of the canonical VAIK motif lysine is compensated by K39, located N-terminal to αC. Evolutionary analysis suggests that degradation of active site motifs in metazoan ULK4 has co-occurred with an ULK4-specific activation loop, which stabilizes the C helix. In addition, cellular interaction studies using BioID and biochemical validation data revealed high confidence interactors of the pseudokinase and armadillo repeat domains. Many of the identified ULK4 interaction partners were centrosomal and tubulin-associated proteins and several active kinases suggesting interesting regulatory roles for ULK4.
中文翻译:

假激酶 Unc-51 样激酶 4 的核苷酸结合、进化见解和相互作用伙伴。
Unc-51 样激酶 4 (ULK4) 是一种假激酶,与多种疾病的发生有关。尽管缺乏激酶中 ATP 结合所需的序列基序,ULK4 仍然紧密结合 ATP,并且辅助因子的存在是 ULK4 结构稳定性所必需的。在这里,我们展示了 ULK4-ATPγS 复合物的高分辨率结构,揭示了一种非常不寻常的 ATP 结合模式,其中典型 VAIK 基序赖氨酸的缺乏由位于 αC N 端的 K39 补偿。进化分析表明,后生动物 ULK4 中活性位点基序的降解与 ULK4 特异性激活环同时发生,从而稳定了 C 螺旋。此外,使用 BioID 和生化验证数据进行的细胞相互作用研究揭示了假激酶和犰狳重复结构域的高置信度相互作用因子。许多已鉴定的 ULK4 相互作用伙伴是中心体和微管蛋白相关蛋白以及几种活性激酶,表明 ULK4 具有有趣的调节作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号