当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isobaric Vapor–Liquid Equilibrium of Binary Systems (Isopropyl Acetate/Isopropyl Alcohol + Dibutyl Ether/ Anisole) at 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.jced.0c00204 Wenjun Wang 1 , Yi Zhang 1 , Tao Zhang 1 , Jun Gao 1 , Dongmei Xu 1 , Lianzheng Zhang 1, 2 , Yinglong Wang 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.jced.0c00204 Wenjun Wang 1 , Yi Zhang 1 , Tao Zhang 1 , Jun Gao 1 , Dongmei Xu 1 , Lianzheng Zhang 1, 2 , Yinglong Wang 3
Affiliation
|
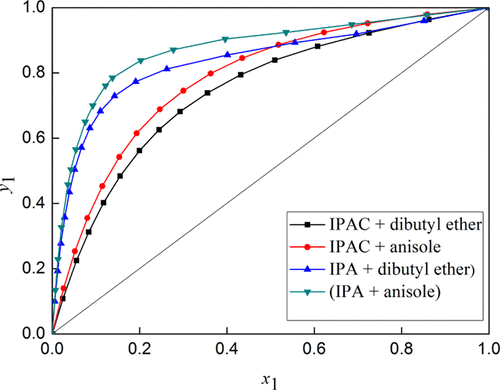
Isopropyl acetate and isopropyl alcohol were azeotropic mixtures with a minimum boiling point. To break the azeotrope of the above binary system and separate it, dibutyl ether and anisole were chosen as the extractive solvents in this corresponding extractive distillation process. Therefore, the vapor–liquid equilibrium (VLE) measurements for (isopropyl alcohol + dibutyl ether), (isopropyl alcohol + anisole), (isopropyl acetate + dibutyl ether), and (isopropyl acetate + anisole) at 101.3 kPa were achieved by a modified Rose type recirculating still. The VLE data were checked by the van Ness test and pure component consistency test. The NRTL, UNIQUAC, and Wilson models were applied to correlate the determined VLE data. Furthermore, the suitability of entrainers was investigated by relative volatility and the effect of selected entrainers on the azeotrope. After that, the obtained binary interaction parameters can provide a basis for the simulation and optimization of the extractive distillation process.
中文翻译:

二元体系的等压蒸气-液体平衡(乙酸异丙酯/异丙醇+二丁醚/苯甲醚)在101.3 kPa
乙酸异丙酯和异丙醇是具有最小沸点的共沸混合物。为了打破上述二元体系的共沸物并将其分离,在该相应的萃取蒸馏过程中,选择二丁醚和茴香醚作为萃取溶剂。因此,通过改进的方法,可以在101.3 kPa下测量(异丙醇+二丁醚),(异丙醇+茴香醚),(乙酸异丙酯+二丁醚)和(乙酸异丙酯+苯甲醚)的气液平衡(VLE)。玫瑰型仍在循环。VLE数据通过van Ness测试和纯组分一致性测试进行检查。应用了NRTL,UNIQUAC和Wilson模型来关联确定的VLE数据。此外,通过相对挥发性和所选夹带剂对共沸物的影响,研究了夹带剂的适用性。此后,所获得的二元相互作用参数可以为萃取蒸馏过程的模拟和优化提供基础。
更新日期:2020-09-10
中文翻译:

二元体系的等压蒸气-液体平衡(乙酸异丙酯/异丙醇+二丁醚/苯甲醚)在101.3 kPa
乙酸异丙酯和异丙醇是具有最小沸点的共沸混合物。为了打破上述二元体系的共沸物并将其分离,在该相应的萃取蒸馏过程中,选择二丁醚和茴香醚作为萃取溶剂。因此,通过改进的方法,可以在101.3 kPa下测量(异丙醇+二丁醚),(异丙醇+茴香醚),(乙酸异丙酯+二丁醚)和(乙酸异丙酯+苯甲醚)的气液平衡(VLE)。玫瑰型仍在循环。VLE数据通过van Ness测试和纯组分一致性测试进行检查。应用了NRTL,UNIQUAC和Wilson模型来关联确定的VLE数据。此外,通过相对挥发性和所选夹带剂对共沸物的影响,研究了夹带剂的适用性。此后,所获得的二元相互作用参数可以为萃取蒸馏过程的模拟和优化提供基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号