当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Michael Addition as a Method for Polyisobutylene Chain-End Functionalization.
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2020-08-16 , DOI: 10.1002/marc.202000382 Ihor Kulai 1 , Andrii Karpus 2 , David E Bergbreiter 3 , Mohammed Al-Hashimi 1 , Hassan S Bazzi 1, 4
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2020-08-16 , DOI: 10.1002/marc.202000382 Ihor Kulai 1 , Andrii Karpus 2 , David E Bergbreiter 3 , Mohammed Al-Hashimi 1 , Hassan S Bazzi 1, 4
Affiliation
|
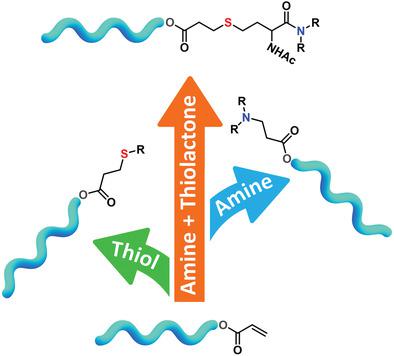
Functionalization of polyolefins, in particular polyisobutylene, remains a relatively unexplored application for the Michael reaction. This work evaluates the potential of polyisobutylene acrylate (PIBA) chain‐end modification via organocatalyzed thiol‐Michael and aza‐Michael additions. A series of chain‐end functional polyisobutylene oligomers are prepared using “click” reactions of thiols or amines to PIBA in the presence of 0.02 equivalents of organocatalyst. Reaction kinetics and chain‐end transformations are monitored using NMR spectroscopy and the macromolecular products are characterized by size exclusion chromatography. Further potential of this synthetic strategy is illustrated by thiol‐Michael addition of thiols formed in situ via nucleophilic thiolactone ring opening. The obtained results provide an efficient method for the preparation of functional polyisobutylene oligomers that can be utilized in a broad range of potential applications.
中文翻译:

有机催化迈克尔加成作为聚异丁烯链末端官能化的方法。
聚烯烃,特别是聚异丁烯的官能化对于迈克尔反应仍然是一个尚未开发的应用。这项工作评估了通过有机催化的硫醇-迈克尔和氮杂-迈克尔加成物对聚异丁烯丙烯酸酯(PIBA)链端修饰的潜力。使用硫醇或胺与PIBA的“点击”反应制备了一系列链端官能聚异丁烯低聚物在0.02当量的有机催化剂存在下。使用NMR光谱监测反应动力学和链端转化,并通过尺寸排阻色谱对大分子产物进行表征。通过亲核硫代内酯开环原位形成的硫醇的硫醇-迈克尔加成反应说明了这种合成策略的进一步潜力。所获得的结果提供了一种制备功能性聚异丁烯低聚物的有效方法,该方法可用于广泛的潜在应用中。
更新日期:2020-09-08
中文翻译:

有机催化迈克尔加成作为聚异丁烯链末端官能化的方法。
聚烯烃,特别是聚异丁烯的官能化对于迈克尔反应仍然是一个尚未开发的应用。这项工作评估了通过有机催化的硫醇-迈克尔和氮杂-迈克尔加成物对聚异丁烯丙烯酸酯(PIBA)链端修饰的潜力。使用硫醇或胺与PIBA的“点击”反应制备了一系列链端官能聚异丁烯低聚物在0.02当量的有机催化剂存在下。使用NMR光谱监测反应动力学和链端转化,并通过尺寸排阻色谱对大分子产物进行表征。通过亲核硫代内酯开环原位形成的硫醇的硫醇-迈克尔加成反应说明了这种合成策略的进一步潜力。所获得的结果提供了一种制备功能性聚异丁烯低聚物的有效方法,该方法可用于广泛的潜在应用中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号