Developmental Cell ( IF 10.7 ) Pub Date : 2020-08-17 , DOI: 10.1016/j.devcel.2020.07.015 Jenna M Frame 1 , Caroline Kubaczka 1 , Timothy L Long 1 , Virginie Esain 1 , Rebecca A Soto 2 , Mariam Hachimi 1 , Ran Jing 1 , Arkadi Shwartz 3 , Wolfram Goessling 4 , George Q Daley 5 , Trista E North 6
|
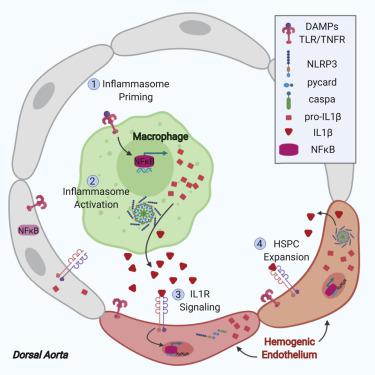
Embryonic hematopoietic stem and progenitor cells (HSPCs) robustly proliferate while maintaining multilineage potential in vivo; however, an incomplete understanding of spatiotemporal cues governing their generation has impeded robust production from human induced pluripotent stem cells (iPSCs) in vitro. Using the zebrafish model, we demonstrate that NLRP3 inflammasome-mediated interleukin-1-beta (IL1β) signaling drives HSPC production in response to metabolic activity. Genetic induction of active IL1β or pharmacologic inflammasome stimulation increased HSPC number as assessed by in situ hybridization for runx1/cmyb and flow cytometry. Loss of inflammasome components, including il1b, reduced CD41+ HSPCs and prevented their expansion in response to metabolic cues. Cell ablation studies indicated that macrophages were essential for initial inflammasome stimulation of Il1rl1+ HSPCs. Significantly, in human iPSC-derived hemogenic precursors, transient inflammasome stimulation increased multilineage hematopoietic colony-forming units and T cell progenitors. This work establishes the inflammasome as a conserved metabolic sensor that expands HSPC production in vivo and in vitro.
中文翻译:

炎症小体活性的代谢调节控制胚胎造血干细胞和祖细胞的产生。
胚胎造血干细胞和祖细胞 (HSPCs) 在保持体内多向潜能的同时强劲增殖;然而,对控制其产生的时空线索的不完全理解阻碍了体外人类诱导多能干细胞 (iPSCs) 的稳健生产。使用斑马鱼模型,我们证明 NLRP3 炎症小体介导的白细胞介素 1-β (IL1β) 信号驱动 HSPC 产生以响应代谢活动。通过runx1/cmyb和流式细胞术的原位杂交评估,活性 IL1β 的遗传诱导或药理学炎症小体刺激增加了 HSPC 数量。炎性体成分的丧失,包括il1b,降低了 CD41+ HSPCs 并阻止它们响应代谢线索的扩张。细胞消融研究表明巨噬细胞对于 Il1rl1 + HSPC 的初始炎症小体刺激至关重要。值得注意的是,在人类 iPSC 衍生的造血前体中,瞬时炎症小体刺激增加了多系造血集落形成单位和 T 细胞祖细胞。这项工作将炎性体确立为一种保守的代谢传感器,可在体内和体外扩大 HSPC 的产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号