Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-08-16 , DOI: 10.1016/j.bbamem.2020.183439 Emilie Segura 1 , Amrit Mehta 2 , Mireille Marsolais 3 , Xin R Quan 2 , Juan Zhao 3 , Rémy Sauvé 4 , J David Spafford 2 , Lucie Parent 1
|
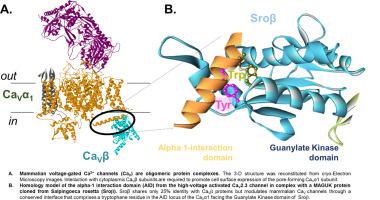
Eukaryote voltage-gated Ca2+ channels of the CaV2 channel family are hetero-oligomers formed by the pore-forming CaVα1 protein assembled with auxiliary CaVα2δ and CaVβ subunits. CaVβ subunits are formed by a Src homology 3 (SH3) domain and a guanylate kinase (GK) domain connected through a HOOK domain. The GK domain binds a conserved cytoplasmic region of the pore-forming CaVα1 subunit referred as the “AID”. Herein we explored the phylogenetic and functional relationship between CaV channel subunits in distant eukaryotic organisms by investigating the function of a MAGUK protein (XM_004990081) cloned from the choanoflagellate Salpingoeca rosetta (Sro). This MAGUK protein (Sroβ) features SH3 and GK structural domains with a 25% primary sequence identity to mammalian CaVβ. Recombinant expression of its cDNA with mammalian high-voltage activated Ca2+ channel CaV2.3 in mammalian HEK cells produced robust voltage-gated inward Ca2+ currents with typical activation and inactivation properties. Like CaVβ, Sroβ prevents fast degradation of total CaV2.3 proteins in cycloheximide assays. The three-dimensional homology model predicts an interaction between the GK domain of Sroβ and the AID motif of the pore-forming CaVα1 protein. Substitution of AID residues Trp (W386A) and Tyr (Y383A) significantly impaired co-immunoprecipitation of CaV2.3 with Sroβ and functional upregulation of CaV2.3 currents. Likewise, a 6-residue deletion within the GK domain of Sroβ, similar to the locus found in mammalian CaVβ, significantly reduced peak current density. Altogether our data demonstrate that an ancestor MAGUK protein reconstitutes the biophysical and molecular features responsible for channel upregulation by mammalian CaVβ through a minimally conserved molecular interface.
中文翻译:

祖先的MAGUK蛋白支持通过保守的CaVβ样界面调节哺乳动物电压门控的Ca2 +通道。
真核生物电压门控的Ca 2+的钙通道V 2通道家族是由孔形成的Ca形成异寡聚体V与辅助的Ca组装α1蛋白V α2δ和Ca V β亚基。的Ca V β亚基由Src同源3(SH3)结构域,并通过HOOK域连接的鸟苷酸激酶(GK)结构域形成。该GK结构域结合的孔形成的Ca的保守胞质区V α1亚单位中被称为“AID”。在这里,我们通过研究从鞭毛鞭毛虫克隆的MAGUK蛋白(XM_004990081)的功能,探索了远距离真核生物中Ca V通道亚基之间的系统发生和功能关系。Salpingoeca rosetta(Sro)。此MAGUK蛋白(Sroβ)设有SH3和GK用25%一级序列同一性的哺乳动物的Ca的结构域V β。在哺乳动物HEK细胞中,其cDNA与哺乳动物高压激活的Ca 2+通道Ca V 2.3的重组表达产生了具有典型激活和失活特性的强大的电压门控内向Ca 2+电流。如Ca V β,Sroβ防止总的Ca的快速降解V在放线菌酮测定法2.3的蛋白质。三维同源模型预测Sroβ的GK结构域与成孔Ca V的AID基序之间的相互作用α1蛋白。替代AID残基Trp(W386A)和Tyr(Y383A)会严重损害Ca V 2.3与Sroβ的共免疫沉淀以及Ca V 2.3电流的功能上调。同样地,Sroβ的GK域内的6个残基的缺失,类似于轨迹在哺乳动物中发现的Ca V β,显著降低的峰值电流密度。总之我们的数据表明,一个祖先MAGUK蛋白重新构成负责信道上调哺乳动物钙生物物理和分子特性V通过最低限度地保守的分子界面β。











































 京公网安备 11010802027423号
京公网安备 11010802027423号