当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkyltriflones in the Ramberg–Bäcklund Reaction: An Efficient and Modular Synthesis of gem-Difluoroalkenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-16 , DOI: 10.1021/jacs.0c07924 Yuuki Maekawa 1, 2 , Masakazu Nambo 2 , Daisuke Yokogawa 3 , Cathleen M Crudden 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-16 , DOI: 10.1021/jacs.0c07924 Yuuki Maekawa 1, 2 , Masakazu Nambo 2 , Daisuke Yokogawa 3 , Cathleen M Crudden 1, 2
Affiliation
|
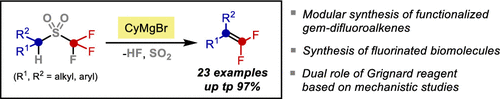
The unprecedented synthesis of gem-difluoroalkenes through the Ramberg-Bäcklund reaction of alkyl triflones is described herein. Structurally diverse, fully-substituted gem-difluoroalkenes that are difficult to prepare by other methods can be easily prepared from readily available triflones by treatment with specific Grignard reagents. Experimental and computational studies provide insight into the unique and critical role of Grignard reagent, which serves both as a base to remove the alpha-proton, and as a Lewis acid to assist C-F bond activation.
中文翻译:

Ramberg-Bäcklund 反应中的烷基三氟乙烯:一种高效、模块化的 gem-二氟烯烃合成
本文描述了通过烷基三氟乙烯的 Ramberg-Bäcklund 反应前所未有的 gem-二氟烯烃合成。通过用特定的格氏试剂处理,可以很容易地从容易获得的三氟酮中制备结构多样、完全取代的偕二氟烯烃,这些烯烃很难通过其他方法制备。实验和计算研究提供了对格氏试剂独特和关键作用的深入了解,该试剂既可作为去除 α-质子的碱,又可作为路易斯酸帮助 CF 键活化。
更新日期:2020-08-16
中文翻译:

Ramberg-Bäcklund 反应中的烷基三氟乙烯:一种高效、模块化的 gem-二氟烯烃合成
本文描述了通过烷基三氟乙烯的 Ramberg-Bäcklund 反应前所未有的 gem-二氟烯烃合成。通过用特定的格氏试剂处理,可以很容易地从容易获得的三氟酮中制备结构多样、完全取代的偕二氟烯烃,这些烯烃很难通过其他方法制备。实验和计算研究提供了对格氏试剂独特和关键作用的深入了解,该试剂既可作为去除 α-质子的碱,又可作为路易斯酸帮助 CF 键活化。







































 京公网安备 11010802027423号
京公网安备 11010802027423号