当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Early detection of prion protein aggregation with a fluorescent pentameric oligothiophene probe using spectral confocal microscopy
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-08-16 , DOI: 10.1111/jnc.15148 Anastasiia Stepanchuk 1 , Waqas Tahir 2 , K Peter R Nilsson 3 , Hermann M Schatzl 2 , Peter K Stys 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-08-16 , DOI: 10.1111/jnc.15148 Anastasiia Stepanchuk 1 , Waqas Tahir 2 , K Peter R Nilsson 3 , Hermann M Schatzl 2 , Peter K Stys 1
Affiliation
|
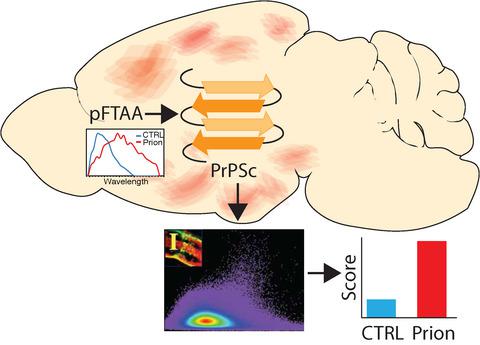
Misfolding of the prion protein (PrP) and templating of its pathological conformation onto cognate proteins causes a number of lethal disorders of central nervous system in humans and animals, such as Creutzfeldt–Jacob disease, chronic wasting disease and bovine spongiform encephalopathy. Structural rearrangement of PrPC into PrPSc promotes aggregation of misfolded proteins into β‐sheet‐rich fibrils, which can be visualized by conformationally sensitive fluorescent probes. Early detection of prion misfolding and deposition might provide useful insights into its pathophysiology. Pentameric formyl thiophene acetic acid (pFTAA) is a novel amyloid probe that was shown to sensitively detect various misfolded proteins, including PrP. Here, we compared sensitivity of pFTAA staining and spectral microscopy with conventional methods of prion detection in mouse brains infected with mouse‐adapted 22L prions. pFTAA bound to prion deposits in mouse brain sections exhibited a red‐shifted fluorescence emission spectrum, which quantitatively increased with disease progression. Small prion deposits were detected as early as 50 days post‐inoculation, well before appearance of clinical signs. Moreover, we detected significant spectral shifts in the greater brain parenchyma as early as 25 days post‐inoculation, rivaling the most sensitive conventional method (real‐time quaking‐induced conversion). These results showcase the potential of pFTAA staining combined with spectral imaging for screening of prion‐infected tissue. Not only does this method have comparable sensitivity to established techniques, it is faster and technically simpler. Finally, this readout provides valuable information about the spatial distribution of prion aggregates across tissue in the earliest stages of infection, potentially providing valuable pathophysiological insight into prion transmission.
中文翻译:

使用光谱共聚焦显微镜用荧光五聚体寡噻吩探针早期检测of病毒蛋白聚集
ion病毒蛋白(PrP)的错误折叠以及将其病理构象模板化到同源蛋白上会导致人和动物的许多中枢神经系统致死性疾病,例如Creutzfeldt–Jacob病,慢性消耗性疾病和牛海绵状脑病。PrP C的结构重排为PrP Sc促进错误折叠的蛋白质聚集到富含β-折叠的原纤维中,这可以通过构象敏感的荧光探针观察到。detection病毒错折叠和沉积的早期检测可能会提供有用的洞察其病理生理学。五聚甲酰基噻吩乙酸(pFTAA)是一种新型淀粉样蛋白探针,已显示出可灵敏地检测各种错误折叠的蛋白质,包括PrP。在这里,我们将pFTAA染色的灵敏度和光谱显微镜与常规的of病毒检测方法在感染了适应小鼠的22L ions病毒的小鼠脑中进行了比较。与小鼠脑部brain病毒沉积物结合的pFTAA表现出红移的荧光发射光谱,其随疾病进展而定量增加。早在接种后50天,即在临床体征出现之前,就发现了小病毒沉积物。此外,早在接种后25天,我们就在较大的脑实质中发现了明显的光谱偏移,可与最灵敏的传统方法(实时震荡诱导的转换)相媲美。这些结果表明,pFTAA染色与光谱成像相结合的潜力可用于筛查感染pr病毒的组织。这种方法不仅具有与现有技术相当的灵敏度,而且速度更快,技术更简单。最后,该读数提供了有关感染早期阶段across病毒聚集体在整个组织中的空间分布的有价值的信息,从而有可能为ion病毒的传播提供有价值的病理生理学见解。与最敏感的传统方法(实时地震诱发的转换)相抗衡。这些结果表明,pFTAA染色与光谱成像相结合的潜力可用于筛查感染pr病毒的组织。这种方法不仅具有与现有技术相当的灵敏度,而且速度更快,技术更简单。最后,该读数提供了有关感染早期阶段across病毒聚集体在整个组织中的空间分布的有价值的信息,从而可能为valuable病毒的传播提供有价值的病理生理学见解。与最敏感的传统方法(实时地震诱发的转换)相抗衡。这些结果表明,pFTAA染色与光谱成像相结合的潜力可用于筛查感染pr病毒的组织。这种方法不仅具有与现有技术相当的灵敏度,而且速度更快,技术更简单。最后,该读数提供了有关感染早期阶段across病毒聚集体在整个组织中的空间分布的有价值的信息,从而可能为valuable病毒的传播提供有价值的病理生理学见解。
更新日期:2020-08-16
中文翻译:

使用光谱共聚焦显微镜用荧光五聚体寡噻吩探针早期检测of病毒蛋白聚集
ion病毒蛋白(PrP)的错误折叠以及将其病理构象模板化到同源蛋白上会导致人和动物的许多中枢神经系统致死性疾病,例如Creutzfeldt–Jacob病,慢性消耗性疾病和牛海绵状脑病。PrP C的结构重排为PrP Sc促进错误折叠的蛋白质聚集到富含β-折叠的原纤维中,这可以通过构象敏感的荧光探针观察到。detection病毒错折叠和沉积的早期检测可能会提供有用的洞察其病理生理学。五聚甲酰基噻吩乙酸(pFTAA)是一种新型淀粉样蛋白探针,已显示出可灵敏地检测各种错误折叠的蛋白质,包括PrP。在这里,我们将pFTAA染色的灵敏度和光谱显微镜与常规的of病毒检测方法在感染了适应小鼠的22L ions病毒的小鼠脑中进行了比较。与小鼠脑部brain病毒沉积物结合的pFTAA表现出红移的荧光发射光谱,其随疾病进展而定量增加。早在接种后50天,即在临床体征出现之前,就发现了小病毒沉积物。此外,早在接种后25天,我们就在较大的脑实质中发现了明显的光谱偏移,可与最灵敏的传统方法(实时震荡诱导的转换)相媲美。这些结果表明,pFTAA染色与光谱成像相结合的潜力可用于筛查感染pr病毒的组织。这种方法不仅具有与现有技术相当的灵敏度,而且速度更快,技术更简单。最后,该读数提供了有关感染早期阶段across病毒聚集体在整个组织中的空间分布的有价值的信息,从而有可能为ion病毒的传播提供有价值的病理生理学见解。与最敏感的传统方法(实时地震诱发的转换)相抗衡。这些结果表明,pFTAA染色与光谱成像相结合的潜力可用于筛查感染pr病毒的组织。这种方法不仅具有与现有技术相当的灵敏度,而且速度更快,技术更简单。最后,该读数提供了有关感染早期阶段across病毒聚集体在整个组织中的空间分布的有价值的信息,从而可能为valuable病毒的传播提供有价值的病理生理学见解。与最敏感的传统方法(实时地震诱发的转换)相抗衡。这些结果表明,pFTAA染色与光谱成像相结合的潜力可用于筛查感染pr病毒的组织。这种方法不仅具有与现有技术相当的灵敏度,而且速度更快,技术更简单。最后,该读数提供了有关感染早期阶段across病毒聚集体在整个组织中的空间分布的有价值的信息,从而可能为valuable病毒的传播提供有价值的病理生理学见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号