当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Eeyarestatin 24 impairs SecYEG‐dependent protein trafficking and inhibits growth of clinically relevant pathogens
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-08-15 , DOI: 10.1111/mmi.14589 Maurice Steenhuis 1 , Gregory M Koningstein 1 , Julia Oswald 2 , Tillman Pick 3 , Sarah O'Keefe 4 , Hans-Georg Koch 2 , Adolfo Cavalié 3 , Roger C Whitehead 5 , Eileithyia Swanton 4 , Stephen High 4 , Joen Luirink 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-08-15 , DOI: 10.1111/mmi.14589 Maurice Steenhuis 1 , Gregory M Koningstein 1 , Julia Oswald 2 , Tillman Pick 3 , Sarah O'Keefe 4 , Hans-Georg Koch 2 , Adolfo Cavalié 3 , Roger C Whitehead 5 , Eileithyia Swanton 4 , Stephen High 4 , Joen Luirink 1
Affiliation
|
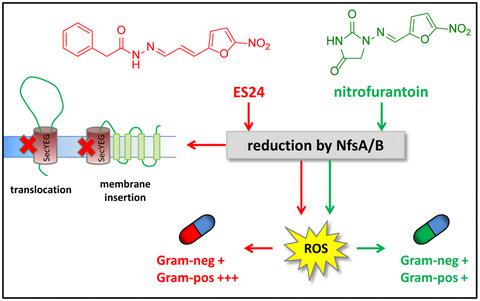
Eeyarestatin 1 (ES1) is an inhibitor of endoplasmic reticulum (ER) associated protein degradation, Sec61‐dependent Ca2+ homeostasis and protein translocation into the ER. Recently, evidence was presented showing that a smaller analog of ES1, ES24, targets the Sec61‐translocon, and captures it in an open conformation that is translocation‐incompetent. We now show that ES24 impairs protein secretion and membrane protein insertion in Escherichia coli via the homologous SecYEG‐translocon. Transcriptomic analysis suggested that ES24 has a complex mode of action, probably involving multiple targets. Interestingly, ES24 shows antibacterial activity toward clinically relevant strains. Furthermore, the antibacterial activity of ES24 is equivalent to or better than that of nitrofurantoin, a known antibiotic that, although structurally similar to ES24, does not interfere with SecYEG‐dependent protein trafficking. Like nitrofurantoin, we find that ES24 requires activation by the NfsA and NfsB nitroreductases, suggesting that the formation of highly reactive nitroso intermediates is essential for target inactivation in vivo.
中文翻译:

Eeyarestatin 24 损害 SecYEG 依赖的蛋白质运输并抑制临床相关病原体的生长
Eeyarestatin 1 (ES1) 是内质网 (ER) 相关蛋白质降解、Sec61 依赖性 Ca 2+稳态和蛋白质易位到 ER 的抑制剂。最近,有证据表明 ES1 的较小类似物 ES24 以 Sec61 易位子为目标,并以无法易位的开放构象捕获它。我们现在表明 ES24 会损害大肠杆菌中的蛋白质分泌和膜蛋白插入通过同源的 SecYEG-易位子。转录组学分析表明 ES24 具有复杂的作用模式,可能涉及多个目标。有趣的是,ES24 对临床相关菌株显示出抗菌活性。此外,ES24 的抗菌活性相当于或优于呋喃妥因,一种已知的抗生素,虽然结构与 ES24 相似,但不会干扰 SecYEG 依赖的蛋白质运输。与呋喃妥因一样,我们发现 ES24 需要被 NfsA 和 NfsB 硝基还原酶激活,这表明高反应性亚硝基中间体的形成对于体内靶标失活至关重要。
更新日期:2020-08-15
中文翻译:

Eeyarestatin 24 损害 SecYEG 依赖的蛋白质运输并抑制临床相关病原体的生长
Eeyarestatin 1 (ES1) 是内质网 (ER) 相关蛋白质降解、Sec61 依赖性 Ca 2+稳态和蛋白质易位到 ER 的抑制剂。最近,有证据表明 ES1 的较小类似物 ES24 以 Sec61 易位子为目标,并以无法易位的开放构象捕获它。我们现在表明 ES24 会损害大肠杆菌中的蛋白质分泌和膜蛋白插入通过同源的 SecYEG-易位子。转录组学分析表明 ES24 具有复杂的作用模式,可能涉及多个目标。有趣的是,ES24 对临床相关菌株显示出抗菌活性。此外,ES24 的抗菌活性相当于或优于呋喃妥因,一种已知的抗生素,虽然结构与 ES24 相似,但不会干扰 SecYEG 依赖的蛋白质运输。与呋喃妥因一样,我们发现 ES24 需要被 NfsA 和 NfsB 硝基还原酶激活,这表明高反应性亚硝基中间体的形成对于体内靶标失活至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号