当前位置:
X-MOL 学术
›
Cytom. Part A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluating spectral cytometry for immune profiling in viral disease.
Cytometry Part A ( IF 2.5 ) Pub Date : 2020-08-16 , DOI: 10.1002/cyto.a.24211 Paula Niewold 1 , Thomas Myles Ashhurst 2 , Adrian Lloyd Smith 2 , Nicholas Jonathan Cole King 1, 2
Cytometry Part A ( IF 2.5 ) Pub Date : 2020-08-16 , DOI: 10.1002/cyto.a.24211 Paula Niewold 1 , Thomas Myles Ashhurst 2 , Adrian Lloyd Smith 2 , Nicholas Jonathan Cole King 1, 2
Affiliation
|
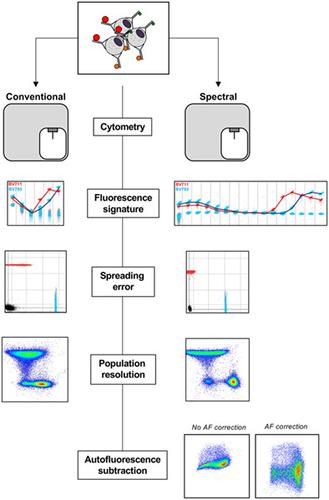
In conventional fluorescence cytometry, each fluorophore present in a panel is measured in a target detector, through the use of wide band‐pass optical filters. In contrast, spectral cytometry uses a large number of detectors with narrow band‐pass filters to measure a fluorophore's signal across the spectrum, creating a more detailed fluorescent signature for each fluorophore. The spectral approach shows promise in adding flexibility to panel design and improving the measurement of fluorescent signal. However, few comparisons between conventional and spectral systems have been reported to date. We therefore sought to compare a modern conventional cytometry system with a modern spectral system, and to assess the quality of resulting datasets from the point of view of a flow cytometry user. Signal intensity, spread, and resolution were compared between the systems. Subsequently, the different methods of separating fluorophore signals were compared, where compensation mathematically separates multiple overlapping fluorophores and unmixing relies on creating a detailed fluorescent signature across the spectrum to separate the fluorophores. Within the spectral data set, signal spread and resolution were comparable between compensation and unmixing. However, for some highly overlapping fluorophores, unmixing resolved the two fluorescence signals where compensation did not. Finally, data from mid‐ to large‐size panels were acquired and were found to have comparable resolution for many fluorophores on both instruments, but reduced levels of spreading error on our spectral system improved signal resolution for a number of fluorophores, compared with our conventional system. Furthermore, autofluorescence extraction on the spectral system allowed for greater population resolution in highly autofluorescent samples. Overall, the implementation of a spectral cytometry approach resulted in data that are comparable to that generated on conventional systems, with a number of potential advantages afforded by the larger number of detectors, and the integration of the spectral unmixing approach. © 2020 International Society for Advancement of Cytometry
中文翻译:

评估用于病毒性疾病免疫分析的光谱细胞术。
在传统的荧光细胞计数中,面板中存在的每个荧光团都在目标检测器中通过使用宽带通滤光器进行测量。相比之下,光谱细胞术使用大量带有窄带通滤波器的检测器来测量整个光谱中荧光团的信号,为每个荧光团创建更详细的荧光特征。光谱方法在增加面板设计的灵活性和改进荧光信号的测量方面显示出前景。然而,迄今为止,很少有关于传统系统和光谱系统之间的比较的报道。因此,我们试图将现代传统细胞术系统与现代光谱系统进行比较,并从流式细胞术用户的角度评估所得数据集的质量。信号强度、传播、和分辨率在系统之间进行了比较。随后,比较了分离荧光团信号的不同方法,其中补偿在数学上分离了多个重叠的荧光团,分离依赖于在整个光谱上创建详细的荧光特征来分离荧光团。在光谱数据集中,补偿和解混之间的信号扩展和分辨率相当。然而,对于一些高度重叠的荧光团,解混解决了补偿没有解决的两个荧光信号。最后,获得了中到大尺寸面板的数据,发现两种仪器上许多荧光团的分辨率相当,但我们光谱系统上的扩散误差水平的降低提高了许多荧光团的信号分辨率,与我们的传统系统相比。此外,光谱系统上的自发荧光提取允许在高度自发荧光的样品中获得更大的群体分辨率。总体而言,光谱细胞计数方法的实施产生的数据与传统系统产生的数据相当,具有大量检测器提供的许多潜在优势,以及光谱分离方法的集成。© 2020 国际细胞计量学促进会 具有由大量探测器提供的许多潜在优势,以及光谱分离方法的集成。© 2020 国际细胞计量学促进会 具有由大量探测器提供的许多潜在优势,以及光谱分离方法的集成。© 2020 国际细胞计量学促进会
更新日期:2020-08-16
中文翻译:

评估用于病毒性疾病免疫分析的光谱细胞术。
在传统的荧光细胞计数中,面板中存在的每个荧光团都在目标检测器中通过使用宽带通滤光器进行测量。相比之下,光谱细胞术使用大量带有窄带通滤波器的检测器来测量整个光谱中荧光团的信号,为每个荧光团创建更详细的荧光特征。光谱方法在增加面板设计的灵活性和改进荧光信号的测量方面显示出前景。然而,迄今为止,很少有关于传统系统和光谱系统之间的比较的报道。因此,我们试图将现代传统细胞术系统与现代光谱系统进行比较,并从流式细胞术用户的角度评估所得数据集的质量。信号强度、传播、和分辨率在系统之间进行了比较。随后,比较了分离荧光团信号的不同方法,其中补偿在数学上分离了多个重叠的荧光团,分离依赖于在整个光谱上创建详细的荧光特征来分离荧光团。在光谱数据集中,补偿和解混之间的信号扩展和分辨率相当。然而,对于一些高度重叠的荧光团,解混解决了补偿没有解决的两个荧光信号。最后,获得了中到大尺寸面板的数据,发现两种仪器上许多荧光团的分辨率相当,但我们光谱系统上的扩散误差水平的降低提高了许多荧光团的信号分辨率,与我们的传统系统相比。此外,光谱系统上的自发荧光提取允许在高度自发荧光的样品中获得更大的群体分辨率。总体而言,光谱细胞计数方法的实施产生的数据与传统系统产生的数据相当,具有大量检测器提供的许多潜在优势,以及光谱分离方法的集成。© 2020 国际细胞计量学促进会 具有由大量探测器提供的许多潜在优势,以及光谱分离方法的集成。© 2020 国际细胞计量学促进会 具有由大量探测器提供的许多潜在优势,以及光谱分离方法的集成。© 2020 国际细胞计量学促进会











































 京公网安备 11010802027423号
京公网安备 11010802027423号