Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-16 , DOI: 10.1016/j.tet.2020.131493 Yoshikazu Horino , Mayo Ishibashi , Kosuke Nakasai , Toshinobu Korenaga
|
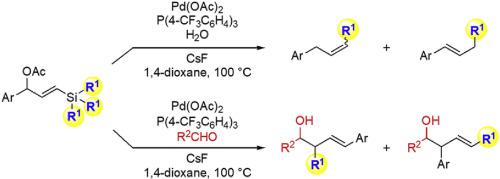
The palladium-catalyzed reaction of γ-silylated allyl acetates with water in the presence of CsF induces a previously unprecedented 1,2-shift of a substituent on silicon to produce allylsilanes in situ. The catalytic activity of the palladium increased when using an electron-poor phosphine ligand possessing fluorinated substituents. Further investigation of the reaction revealed that the approximate order of the migratory aptitude of groups from silicon was PhC≡C, allyl > Bn > Ph, vinyl > alkyl (Me, Et). A density functional theory study was employed to explore the reaction mechanism. Finally, the Hosomi–Sakurai-type allylation of aldehydes with in situ-generated α,γ-disubstituted allylsilanes was also investigated.
中文翻译:

通过硅上取代基的1,2-转移进行的γ-硅烷化乙酸烯丙酯的钯催化反应
在CsF存在下,钯催化的γ-甲硅烷基化的乙酸烯丙酯与水的反应会导致硅上取代基发生前所未有的1,2-移位,从而原位生成烯丙基硅烷。当使用具有氟化取代基的贫电子膦配体时,钯的催化活性增加。对该反应的进一步研究表明,基团从硅的迁移能力的近似顺序为:PhC≡C,烯丙基> Bn> Ph,乙烯基>烷基(Me,Et)。利用密度泛函理论研究了反应机理。最后,还研究了Hosomi-Sakurai型醛与原位生成的α,γ-二取代烯丙基硅烷的烯丙基化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号