Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-08-16 , DOI: 10.1016/j.actbio.2020.08.015 Thomas Hamada 1 , Julie L N Dubois 2 , Valérie Bellamy 3 , Laetitia Pidial 3 , Albert Hagège 4 , Maria N Pereira 2 , Philippe Menasché 5
|
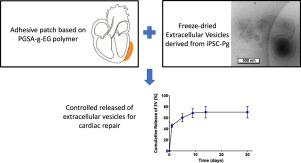
Cell therapy to restore cardiac function in chronic heart failure has been extensively studied. However, its therapeutic value is limited due to poor cell engraftment and survival and the therapeutic outcomes have been attributed to paracrine secretions such as extracellular vesicles (EV). The direct use of EV is an attractive therapeutic strategy and it has been shown that the kinetics of delivery of the EV to the targeted tissue may impact the outcomes. However, there are currently no technologies to deliver EV to the heart in a controlled and tunable manner. The objective of this study was to design a controlled release system, based on a photocurable adhesive polymer, to locally deliver EV to the cardiac tissue. We have first demonstrated that the adhesive polymer, PGSA-g-EG, did not impact the EV bioactivity in vitro and was biocompatible in vivo when tested in a rat model. Importantly, the polymer remained attached to the heart surface for at least 1 month. We have then evaluated and optimized the in vitro release kinetics of the EV from the PGSA-g-EG polymer. Freeze-dried EV formulations were developed to tune the release kinetics and maximize the loading in the polymeric material. Moreover, despite the instability of the EV in aqueous medium at 37°C, the PGSA-g-EG polymer was able to release bioactive EV for at least 14 days. Overall, these results suggest that the PGSA-g-EG is a suitable material to promote the controlled delivery of bioactive EV over an extended period of time.
Statement of Significance
Extracellular vesicles (EV) are an investigational class of therapeutics that has shown promise to restore cardiac function following an ischemic event. Furthermore, its translation to the clinics is expected to pose less regulatory challenges than cell-based therapies. However, EV therapeutic outcomes are likely to be impacted by the route of administration and the kinetics of delivery to the target tissue. Therefore, there is a need for biomaterial-based technologies to deliver, in a controlled and tunable manner, EV to the heart. The present study describes the use of PGSA-g-EG polymer as an adhesive cardiac patch with potential to enable the controlled delivery of bioactive EV over an extended period of time to the cardiac tissue.
中文翻译:

从聚(癸二酸甘油酯)丙烯酸酯基聚合物中进行心脏修复的细胞外小泡的体外控制释放。
在慢性心力衰竭中恢复心脏功能的细胞疗法已被广泛研究。然而,由于不良的细胞植入和存活,其治疗价值受到限制,并且治疗结果已归因于旁分泌分泌物,例如细胞外囊泡(EV)。EV的直接使用是一种有吸引力的治疗策略,并且已经表明,将EV输送到目标组织的动力学可能会影响结果。但是,目前尚无以可控和可调的方式将EV输送到心脏的技术。这项研究的目的是设计一种基于光固化粘合剂聚合物的控释系统,以将EV局部递送至心脏组织。我们首先证明了粘性聚合物PGSA-g-EG不会影响EV的体外生物活性并且在大鼠模型中进行测试时具有体内生物相容性。重要的是,聚合物保持附着在心脏表面至少1个月。然后,我们评估并优化了PGSA-g-EG聚合物的EV体外释放动力学。开发了冻干的电动汽车配方,以调节释放动力学并使聚合物材料中的负载最大化。而且,尽管EV在37℃的水性介质中不稳定,但PGSA-g-EG聚合物能够释放生物活性EV至少14天。总体而言,这些结果表明,PGSA-g-EG是一种合适的材料,可在较长的时间内促进生物活性EV的受控递送。
重要声明
细胞外囊泡(EV)是一类研究性的治疗药物,已显示有望在缺血事件后恢复心脏功能。此外,与基于细胞的疗法相比,将其翻译为临床有望带来更少的监管挑战。但是,电动汽车的治疗结果可能会受到给药途径和向靶组织的输送动力学的影响。因此,需要基于生物材料的技术以可控和可调谐的方式将EV传递至心脏。本研究描述了使用PGSA-g-EG聚合物作为心脏粘连贴剂的潜力,该潜力具有使生物活性EV在延长的时间段内可控地递送至心脏组织的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号