当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Simulations of Aqueous Nonionic Surfactants on a Carbonate Surface.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.jpcb.0c03997 Soumik Das 1 , Fardin Khabaz 2, 3 , Quoc Nguyen 4 , Roger T Bonnecaze 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.jpcb.0c03997 Soumik Das 1 , Fardin Khabaz 2, 3 , Quoc Nguyen 4 , Roger T Bonnecaze 1
Affiliation
|
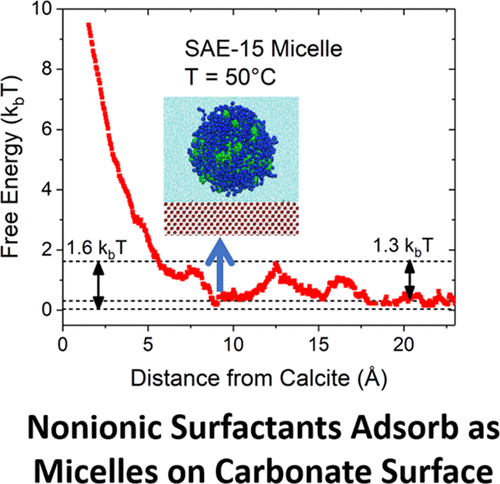
The interactions and structure of secondary alcohol ethoxylates with 15 and 40 ethoxylate units in water near a calcite surface are studied. It is found that water binds preferentially to the calcite surface. Prediction of the free-energy landscape for surfactant molecules shows that single-surfactant molecules do not adsorb because they cannot get close enough to the surface because of the water layer for attractive ethoxylate–calcite or dispersion interactions to be significant. Micelles can adsorb onto the surface even with the intervening water layer because of the integrative effect of the attractive interactions of all the surfactant molecules. Adsorption is found to increase because of the closer proximity of the micelles to the surface due to a weakened water layer at higher temperatures. The free-energy well and barrier values are used to estimate surface to bulk partition coefficients for different surfactants and temperatures, and qualitative agreement is found with experimental observations. The combined effect of surfactant–water and surfactant–solid interactions is found to be responsible for an increased adsorption for nonionic surfactants as the system approaches the cloud point.
中文翻译:

碳酸盐表面上的水性非离子表面活性剂的分子动力学模拟。
研究了方解石表面附近水中具有15和40个乙氧基化物单元的仲醇乙氧基化物的相互作用和结构。发现水优先与方解石表面结合。对表面活性剂分子的自由能态势的预测表明,单表面活性剂分子不会吸附,因为它们不能充分靠近表面,因为水层对乙氧基化物-方解石或分散体的吸引作用非常明显。由于所有表面活性剂分子的吸引力相互作用的整合作用,即使在中间有水层的情况下,胶束也可以吸附到表面上。由于在高温下水层变弱,使胶束更靠近表面,因此发现吸附增加。使用自由能阱和势垒值来估算不同表面活性剂和温度下的表面-本体分配系数,并且与实验观察结果发现定性一致。当系统接近浊点时,发现表面活性剂-水和表面活性剂-固体相互作用的共同作用导致非离子表面活性剂的吸附增加。
更新日期:2020-09-18
中文翻译:

碳酸盐表面上的水性非离子表面活性剂的分子动力学模拟。
研究了方解石表面附近水中具有15和40个乙氧基化物单元的仲醇乙氧基化物的相互作用和结构。发现水优先与方解石表面结合。对表面活性剂分子的自由能态势的预测表明,单表面活性剂分子不会吸附,因为它们不能充分靠近表面,因为水层对乙氧基化物-方解石或分散体的吸引作用非常明显。由于所有表面活性剂分子的吸引力相互作用的整合作用,即使在中间有水层的情况下,胶束也可以吸附到表面上。由于在高温下水层变弱,使胶束更靠近表面,因此发现吸附增加。使用自由能阱和势垒值来估算不同表面活性剂和温度下的表面-本体分配系数,并且与实验观察结果发现定性一致。当系统接近浊点时,发现表面活性剂-水和表面活性剂-固体相互作用的共同作用导致非离子表面活性剂的吸附增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号