当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen-Bonding-Promoted Cascade Rearrangement Involving the Enlargement of Two Rings: Efficient Access to Polycyclic Quinoline Derivatives.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-15 , DOI: 10.1002/anie.202008110 Wen-Bin Cao 1 , Shijun Li 2 , Meng-Meng Xu 1 , Haiyan Li 3 , Xiao-Ping Xu 1 , Yu Lan 2, 4 , Shun-Jun Ji 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-15 , DOI: 10.1002/anie.202008110 Wen-Bin Cao 1 , Shijun Li 2 , Meng-Meng Xu 1 , Haiyan Li 3 , Xiao-Ping Xu 1 , Yu Lan 2, 4 , Shun-Jun Ji 1
Affiliation
|
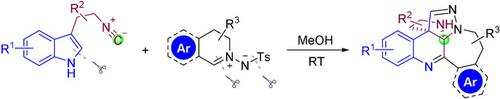
An efficient cascade reaction of tryptamine‐derived isocyanides with C,N‐cyclic azomethine imines is described. The polycyclic pyrrolo[2,3‐c]quinoline derivatives, which benefited from rearrangement process driven by hydrogen bonding, could be directly assembled in moderate to good yields (40–87 %) under metal‐free and mild conditions. This transformation involved four new heterocyclic rings formations and uniquely, ring opening of indole as well as ring expansion of C,N‐cyclic azomethine imine. Both experimental and DFT studies provided guidance on the in‐depth insight into the reaction pathways and hydrogen bonding was identified to lower the free energy barrier in transition states. This work constitutes a rare example of tryptamine‐derived isocyanide‐based cascade reactions, and potentially could be a powerful synthetic strategy for accessing polycyclic analogues involved in natural products.
中文翻译:

氢键键合的级联重排,涉及两个环的扩大:有效获取多环喹啉衍生物。
描述了一种由色胺衍生的异氰酸酯与C,N-环偶氮甲亚胺的有效级联反应。多环吡咯并[2,3-c]喹啉衍生物得益于氢键驱动的重排过程,可以在无金属和温和条件下以中等到良好的产率(40-87%)直接组装。这种转化涉及四个新的杂环形成,并且独特地是吲哚的开环以及C,N-环偶氮甲亚胺亚胺的环扩展。实验研究和DFT研究都为深入了解反应途径提供了指导,并发现氢键可降低过渡态的自由能垒。这项工作是基于色胺的异氰酸酯级联反应的罕见例子,
更新日期:2020-08-15
中文翻译:

氢键键合的级联重排,涉及两个环的扩大:有效获取多环喹啉衍生物。
描述了一种由色胺衍生的异氰酸酯与C,N-环偶氮甲亚胺的有效级联反应。多环吡咯并[2,3-c]喹啉衍生物得益于氢键驱动的重排过程,可以在无金属和温和条件下以中等到良好的产率(40-87%)直接组装。这种转化涉及四个新的杂环形成,并且独特地是吲哚的开环以及C,N-环偶氮甲亚胺亚胺的环扩展。实验研究和DFT研究都为深入了解反应途径提供了指导,并发现氢键可降低过渡态的自由能垒。这项工作是基于色胺的异氰酸酯级联反应的罕见例子,











































 京公网安备 11010802027423号
京公网安备 11010802027423号