NeuroToxicology ( IF 3.4 ) Pub Date : 2020-08-15 , DOI: 10.1016/j.neuro.2020.08.001 J K Akintunde 1 , T E Akintola 1 , G O Adenuga 1 , Z A Odugbemi 2 , R O Adetoye 1 , O G Akintunde 3
|
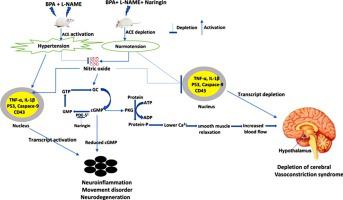
We examined whether active fruit naringin can reduce the risk of BPA-mediated neurotoxicity in L-NAME induced hypertensive rats and whether the modulation could be linked to improvement of brain NO signaling. Male albino rats were randomly distributed into eight (n = 7) groups. Group I was control animals, Group II was orally-treated with L-NAME, Group III was orally treated with 100 mg/kg BPA, Group IV was orally-treated with L-NAME +100 mg/kg BPA. Group V was orally-administered with L-NAME +80 mg/kg NAR. Group VI was orally-administered with 100 mg/kg BPA +80 mg/kg NAR. Group VII was orally-administered with L-NAME+100 mg/kg BPA +80 mg/kg NAR. Lastly, group VIII was orally-treated with 80 mg/kg NAR. The treatment lasted for 14 days. Sub-acute exposure to L-NAME and BPA induced hypertension and mediated-neuroinflammation at CA-2 and CA-4 of hippocampus cells. It was evident by increase in PDE-51 and enzymes of ATP hydrolysis (ATPase, ADPase and AMPase) with corresponding upsurge in cholinergic (AChE and BuChE), dopaminergic (MAO-A) and adenosinergic (ADA) enzymes as well as movement disorder. The hypertensive-mediated neurotoxicity was related to alteration of NO signaling and higher release of pro-inflammatory cytokines (TNF-α and IL-1β), apoptotic proteins (P53 and caspace-9) and facilitated entry of T-lymphocytes (CD43+) into CNS through blood brain barrier potentiated by antigen presenting cells. Hence, these features of BPA-mediated neurotoxicity in L-NAME induced hypertensive rats were prohibited by co-administration of NAR through production of neuro-inflammatory mediators, stabilizing neurotransmitter enzymes, normalizing NO signaling and improving brain histology.
中文翻译:

柚皮苷通过消除脑核苷酸消耗、氧化损伤和神经炎症来减轻双酚 A 介导的高血压大鼠神经毒性。
我们检查了活性水果柚皮苷是否可以降低 L-NAME 诱导的高血压大鼠中 BPA 介导的神经毒性的风险,以及这种调节是否与脑 NO 信号传导的改善有关。雄性白化大鼠随机分为八组(n = 7)。I组是对照动物,II组用L-NAME口服治疗,III组用100mg/kg BPA口服治疗,IV组用L-NAME+100mg/kg BPA口服治疗。V 组口服给药 L-NAME +80 mg/kg NAR。VI 组口服给药 100 mg/kg BPA +80 mg/kg NAR。第七组口服给予 L-NAME+100 mg/kg BPA +80 mg/kg NAR。最后,第 VIII 组口服 80 mg/kg NAR。治疗持续了14天。亚急性暴露于 L-NAME 和 BPA 诱导高血压和介导海马细胞 CA-2 和 CA-4 的神经炎症。PDE-5 的增加很明显1和 ATP 水解酶(ATPase、ADPase 和 AMPase)以及相应的胆碱能(AChE 和 BuChE)、多巴胺能(MAO-A)和腺苷能(ADA)酶以及运动障碍。高血压介导的神经毒性与 NO 信号传导的改变和促炎细胞因子(TNF-α 和 IL-1β)、凋亡蛋白(P53 和 caspace-9)的释放以及促进 T 淋巴细胞的进入(CD43 +)有关通过抗原呈递细胞增强的血脑屏障进入中枢神经系统。因此,通过产生神经炎症介质、稳定神经递质酶、使 NO 信号正常化和改善脑组织学,共同施用 NAR 来抑制 L-NAME 诱导的高血压大鼠中 BPA 介导的神经毒性的这些特征。









































 京公网安备 11010802027423号
京公网安备 11010802027423号