当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-range PEG stapling: macrocyclization for increased protein conformational stability and resistance to proteolysis
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-08-13 , DOI: 10.1039/d0cb00075b Qiang Xiao 1 , Dallin S Ashton 1 , Zachary B Jones 1 , Katherine P Thompson 1 , Joshua L Price 1
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-08-13 , DOI: 10.1039/d0cb00075b Qiang Xiao 1 , Dallin S Ashton 1 , Zachary B Jones 1 , Katherine P Thompson 1 , Joshua L Price 1
Affiliation
|
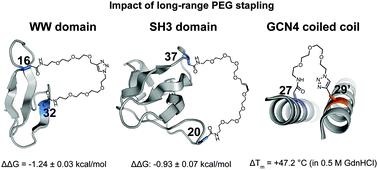
We previously showed that long-range stapling of two Asn-linked O-allyl PEG oligomers via olefin metathesis substantially increases the conformational stability of the WW domain through an entropic effect. The impact of stapling was more favorable when the staple connected positions that were far apart in primary sequence but close in the folded tertiary structure. Here we validate these criteria by identifying new stabilizing PEG-stapling sites within the WW domain and the SH3 domain, both β-sheet proteins. We find that stapling via olefin metathesis vs. the copper(I)-catalyzed azide/alkyne cycloaddition (CuAAC) results in similar energetic benefits, suggesting that olefin and triazole staples can be used interchangeably. Proteolysis assays of selected WW variants reveal that the observed staple-based increases in conformational stability lead to enhanced proteolytic resistance. Finally, we find that an intermolecular staple dramatically increases the quaternary structural stability of an α-helical GCN4 coiled-coil heterodimer.
中文翻译:

长距离 PEG 装订:大环化以增加蛋白质构象稳定性和抗蛋白酶解性
我们之前表明,通过烯烃复分解作用对两个 Asn 连接的O-烯丙基 PEG 低聚物进行长距离缝合,通过熵效应显着增加了 WW 结构域的构象稳定性。当一级序列相距较远但在折叠三级结构中相近的缝合钉连接位置时,缝合的影响更有利。在这里,我们通过在 WW 结构域和 SH3 结构域(均为 β-折叠蛋白)中鉴定新的稳定 PEG 钉合位点来验证这些标准。我们发现通过烯烃复分解的装订与铜(I)-催化的叠氮化物/炔环加成 (CuAAC) 产生类似的能量益处,表明烯烃和三唑主食可以互换使用。选定 WW 变体的蛋白水解分析表明,观察到的基于主食的构象稳定性增加导致蛋白水解抗性增强。最后,我们发现分子间短纤维显着增加了 α-螺旋 GCN4 卷曲螺旋异二聚体的四级结构稳定性。
更新日期:2020-10-08
中文翻译:

长距离 PEG 装订:大环化以增加蛋白质构象稳定性和抗蛋白酶解性
我们之前表明,通过烯烃复分解作用对两个 Asn 连接的O-烯丙基 PEG 低聚物进行长距离缝合,通过熵效应显着增加了 WW 结构域的构象稳定性。当一级序列相距较远但在折叠三级结构中相近的缝合钉连接位置时,缝合的影响更有利。在这里,我们通过在 WW 结构域和 SH3 结构域(均为 β-折叠蛋白)中鉴定新的稳定 PEG 钉合位点来验证这些标准。我们发现通过烯烃复分解的装订与铜(I)-催化的叠氮化物/炔环加成 (CuAAC) 产生类似的能量益处,表明烯烃和三唑主食可以互换使用。选定 WW 变体的蛋白水解分析表明,观察到的基于主食的构象稳定性增加导致蛋白水解抗性增强。最后,我们发现分子间短纤维显着增加了 α-螺旋 GCN4 卷曲螺旋异二聚体的四级结构稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号