当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tartaric acid regulated the advanced synthesis of bismuth-based materials with tunable performance towards the electrocatalytic production of hydrogen peroxide
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-08-14 , DOI: 10.1039/d0ta06466a Paul Morandi 1, 2, 3, 4, 5 , Valerie Flaud 3, 4, 5, 6, 7 , Sophie Tingry 1, 2, 3, 4, 5 , David Cornu 1, 2, 3, 4, 5 , Yaovi Holade 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-08-14 , DOI: 10.1039/d0ta06466a Paul Morandi 1, 2, 3, 4, 5 , Valerie Flaud 3, 4, 5, 6, 7 , Sophie Tingry 1, 2, 3, 4, 5 , David Cornu 1, 2, 3, 4, 5 , Yaovi Holade 1, 2, 3, 4, 5
Affiliation
|
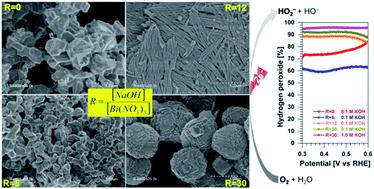
Tartaric acid has emerged as an ecofriendly organic compound for the biogenic preparation of micro- and nano-structured materials. For the synthesis mainly dictated by the coordination chemistry, questions remain open about the impact of the alkalinity on the nature of the resulting material as well as the properties. On the other hand, the design of advanced catalytic materials for the direct production of hydrogen peroxide from air is particularly needed. We report herein new synthesis, physico-chemical and electrocatalytic insights into bismuth-based materials. It was found that tight control of the alkalinity of the synthesis medium through the molar ratio R = [sodium hydroxide]/[bismuth(III) nitrate] leads to the production of a library of bismuth-based materials composed of metallic, (oxy)hydroxide, and oxide structures owing to the bismuth-tartrate complex intermediates. Electroanalytical studies show an oxygen-to-hydrogen peroxide selectivity in 0.1 M KOH of 77, 88 and 92% for R = 0, 12 and 30, respectively. In 1 M KOH, the selectivity is 90 and 96% for R = 12 and 30, respectively. The production rate is 69 ± 3 and 44 ± 3 mol kg−1 cm−2 over 2 h, corresponding to a faradaic efficiency of 92 ± 4 and 62 ± 5% for R = 30 and 12, respectively. These results place the present materials among the best known oxygen-to-hydrogen peroxide reduction catalysts without the need for preparing alloys or specific surface modification. This work contributes to engineering novel catalytic materials for advanced applications of on-site production of valuable chemicals.
中文翻译:

酒石酸调节铋基材料的高级合成,并具有可调节的电催化生产过氧化氢的性能
酒石酸已经成为一种生态友好的有机化合物,用于生物制备微结构和纳米结构的材料。对于主要由配位化学决定的合成,关于碱度对所得材料的性质以及性质的影响仍然存在疑问。另一方面,特别需要设计用于从空气直接生产过氧化氢的高级催化材料。我们在此报告了基于铋的材料的新合成,物理化学和电催化见解。发现通过摩尔比R= [氢氧化钠] / [铋(III)严格控制合成介质的碱度。)[硝酸盐]会导致产生基于铋的酒石酸铋络合物中间体,该数据库由金属,(氧)氢氧化物和氧化物结构组成。电分析研究表明,对于R = 0、12和30,在0.1 M KOH中的氧-过氧化氢选择性分别为77、88和92%。在1 M KOH中,R = 12和30时的选择性分别为90%和96%。生产速率为69±3和44±3摩尔千克-1厘米-2在2小时,这对应于92±4和62±5%的法拉第效率ř= 30和12。这些结果将本材料置于最著名的氧-过氧化氢还原催化剂中,而无需制备合金或进行特定的表面改性。这项工作有助于工程化新型催化材料,用于现场生产有价值的化学品的先进应用。
更新日期:2020-09-22
中文翻译:

酒石酸调节铋基材料的高级合成,并具有可调节的电催化生产过氧化氢的性能
酒石酸已经成为一种生态友好的有机化合物,用于生物制备微结构和纳米结构的材料。对于主要由配位化学决定的合成,关于碱度对所得材料的性质以及性质的影响仍然存在疑问。另一方面,特别需要设计用于从空气直接生产过氧化氢的高级催化材料。我们在此报告了基于铋的材料的新合成,物理化学和电催化见解。发现通过摩尔比R= [氢氧化钠] / [铋(III)严格控制合成介质的碱度。)[硝酸盐]会导致产生基于铋的酒石酸铋络合物中间体,该数据库由金属,(氧)氢氧化物和氧化物结构组成。电分析研究表明,对于R = 0、12和30,在0.1 M KOH中的氧-过氧化氢选择性分别为77、88和92%。在1 M KOH中,R = 12和30时的选择性分别为90%和96%。生产速率为69±3和44±3摩尔千克-1厘米-2在2小时,这对应于92±4和62±5%的法拉第效率ř= 30和12。这些结果将本材料置于最著名的氧-过氧化氢还原催化剂中,而无需制备合金或进行特定的表面改性。这项工作有助于工程化新型催化材料,用于现场生产有价值的化学品的先进应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号