Current Analytical Chemistry ( IF 1.7 ) Pub Date : 2020-08-31 , DOI: 10.2174/1573411015666191010122744 Poonam Rani 1 , Kashmiri Lal 1 , Vikas D. Ghule 2 , Rahul Shrivastava 3
|
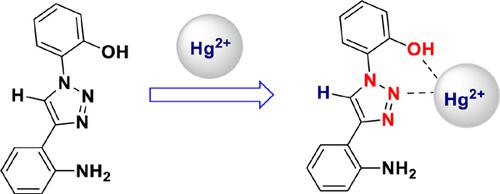
Background: The synthesis of small organic molecules based Hg2+ ions receptors have gained considerable attention because it is one of the most prevalent toxic metals which is continuously discharged into the environment by different natural and industrial activities. 1,4-Disubstituted 1,2,3-triazoles have been reported as good chemosensors for the detection of various metal ions including Hg2+ ions.
Methods: The synthesis of 1,2,3-triazoles (4a-4c) was achieved by Cu(I)-catalyzed azide-alkyne cycloaddition, and their binding affinity towards various metal ions and anions were studied by UVVisible titration experiments. The perchlorate salts of metal ions and tetrabutylammonium salts of anions were utilized for the UV-Visible experiments. DFT studies were performed to understand the binding and mechanism on the sensing of 4a toward Hg2+ using the B3LYP/6-311G(d,p) method for 4a and B3LYP/LANL2DZ for 4a-Hg2+ species on the Gaussian 09W program.
Results: The UV-visible experiments indicated that the compounds 4a-4c show a selective response towards Hg2+ ion in UV-Visible spectra, while other ions did not display such changes in the absorption spectra. The binding stoichiometry was evaluated by Job’s plot which indicated the 1:1 binding stoichiometry between receptors (4a-4c) and Hg2+ ion. The detection limit of 4a, 4b and 4c for the Hg2+ ions was found to be 29.1 nM, 3.5 μM and 1.34 μM, respectively.
Conclusion: Some 1,2,3-triazole derivatives were synthesized (4a-4c) exhibiting high selectively and sensitivity towards Hg2+ ions in preference to other ions. Compound 4a has a low detection limit of 29.1 nM and the binding constant of 2.3×106 M-1. Similarly, 4b and 4c also showed selective sensing towards Hg2+ ions in the μM range. The observed experimental results were corroborated by density functional theory (DFT) calculations.
中文翻译:

基于三唑的化学传感器的绿色合成及其对汞传感的功效
背景:基于Hg2 +离子的有机小分子的合成受到了广泛的关注,因为它是最常见的有毒金属之一,通过不同的自然和工业活动不断排放到环境中。据报道,1,4-二取代的1,2,3-三唑是用于检测包括Hg2 +离子在内的各种金属离子的良好化学传感器。
方法:通过Cu(I)催化的叠氮化物-炔烃环加成反应合成1,2,3-三唑(4a-4c),并通过紫外可见滴定实验研究了它们与各种金属离子和阴离子的结合亲和力。金属离子的高氯酸盐和阴离子的四丁基铵盐被用于紫外可见实验。进行了DFT研究,以了解在高斯09W程序中使用B3LYP / 6-311G(d,p)方法检测4a和4a-Hg2 +物种的B3LYP / LANL2DZ方法对4a对Hg2 +的结合和机理。
结果:紫外可见实验表明,化合物4a-4c在紫外可见光谱中显示出对Hg2 +离子的选择性响应,而其他离子在吸收光谱中未显示出此类变化。结合化学计量由Job's图评估,该图表明受体(4a-4c)和Hg2 +离子之间的1:1结合化学计量。Hg2 +离子的检测限4a,4b和4c分别为29.1 nM,3.5μM和1.34μM。
结论:合成了一些1,2,3-三唑衍生物(4a-4c),这些衍生物具有较高的选择性,并且对Hg2 +离子的敏感性高于其他离子。化合物4a的检测下限为29.1 nM,结合常数为2.3×106 M-1。同样,图4b和图4c也显示了对μM范围内Hg2 +离子的选择性感应。密度泛函理论(DFT)计算证实了观察到的实验结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号