当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism of Ring-Closing Metathesis with a Cationic Molybdenum Imido Alkylidene N-Heterocyclic Carbene Catalyst
Organometallics ( IF 2.5 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.organomet.0c00311 Manoj K. Kesharwani , Iris Elser , Janis V. Musso , Michael R. Buchmeiser , Johannes Kästner
Organometallics ( IF 2.5 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.organomet.0c00311 Manoj K. Kesharwani , Iris Elser , Janis V. Musso , Michael R. Buchmeiser , Johannes Kästner
|
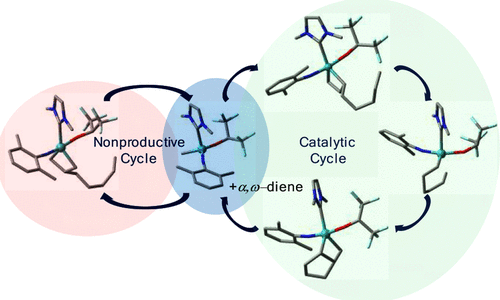
DFT calculations were carried out to explore all relevant pathways for the ring-closing metathesis (RCM) reaction of a cationic molybdenum imido alkylidene N-heterocyclic carbene (NHC) catalyst with α,ω-dienes. Besides the catalytic cycle, which produces the desired cycloalkene in two consecutive metathesis steps, the initiation, a nonproductive cycle, and a degenerative catalytic path were also investigated. On the basis of the approaching face of the diene, two further possibilities were considered for all pathways: syn- and anti-addition. Steric repulsion on the metallacyclobutane ring of the trigonal-bipyramidal (TBP) intermediate appears to be one of the important influencing factors. We found the nonproductive cycle to compete with the catalytic conversion of the substrate. The TBP intermediate formed during the catalytic cycle is energetically lower than the corresponding TBP intermediate of the nonproductive cycle. However, the barriers for the nonproductive cycle are slightly lower than the barrier for the catalytic cycle. Thus, the nonproductive cycle can be expected to be used during catalysis, but it will only have a weak detrimental effect on the turnover rate because it is faster than the catalytic cycle. Of the four different initiation paths, the α-anti-addition path was found to be the energetically most favorable one. Notably, the catalyst was found to be fairly stable against degradation via β-hydride (β-H) transfer to the metal. High activation barriers for the conversion of the TBP intermediate to a square-pyramidal (SP)-based intermediate and a higher energy of a later intermediate suggest a stability of catalyst against degradation. A very high barrier for β-H transfer also disfavors the degradation reaction. The 1H NMR spectrum of the reaction between [Mo(N-2,6-Me2-C6H3)(CHCMe2Ph)(IMes)(OCH(CF3)2)+ B(ArF)4–] and 1,7-octadiene confirms the absence of any β-H transfer.
中文翻译:

阳离子钼亚胺基亚烷基N-杂环卡宾催化剂的闭环复分解反应机理
进行了DFT计算,以探索阳离子钼亚氨基亚烷基N-杂环卡宾(NHC)催化剂与α,ω-二烯的闭环复分解(RCM)反应的所有相关途径。除了在两个连续的复分解步骤中产生所需环烯的催化循环外,还研究了引发,非生产循环和变性催化路径。在二烯接近的基础上,针对所有途径考虑了另外两种可能性:顺式和反式加成。三角双锥体(TBP)中间体的金属环丁烷环上的立体排斥似乎是重要的影响因素之一。我们发现非生产性循环与底物的催化转化竞争。在催化循环期间形成的TBP中间体在能量上低于非生产循环的相应TBP中间体。但是,非生产循环的壁垒略低于催化循环的壁垒。因此,可以预期在催化过程中将使用非生产循环,但是由于它比催化循环更快,因此对周转率的不利影响很小。在四种不同的引发途径中,发现α-反加途径是能量上最有利的途径。值得注意的是 发现该催化剂对于通过β-氢化物(β-H)转移至金属而引起的降解相当稳定。用于TBP中间体转化为基于正棱锥(SP)的中间体的高活化势垒和较高的后期中间体能量提示催化剂对降解的稳定性。β-H转移的很高的阻隔性也不利于降解反应。的[Mo(N-2,6-Me 2 -C 6 H 3)(CHCMe 2 Ph)(IMes)(OCH(CF 3)2)+ B(Ar F)4 – ]之间反应的1 H NMR光谱1,7-辛二烯证实没有任何β-H转移。
更新日期:2020-09-14
中文翻译:

阳离子钼亚胺基亚烷基N-杂环卡宾催化剂的闭环复分解反应机理
进行了DFT计算,以探索阳离子钼亚氨基亚烷基N-杂环卡宾(NHC)催化剂与α,ω-二烯的闭环复分解(RCM)反应的所有相关途径。除了在两个连续的复分解步骤中产生所需环烯的催化循环外,还研究了引发,非生产循环和变性催化路径。在二烯接近的基础上,针对所有途径考虑了另外两种可能性:顺式和反式加成。三角双锥体(TBP)中间体的金属环丁烷环上的立体排斥似乎是重要的影响因素之一。我们发现非生产性循环与底物的催化转化竞争。在催化循环期间形成的TBP中间体在能量上低于非生产循环的相应TBP中间体。但是,非生产循环的壁垒略低于催化循环的壁垒。因此,可以预期在催化过程中将使用非生产循环,但是由于它比催化循环更快,因此对周转率的不利影响很小。在四种不同的引发途径中,发现α-反加途径是能量上最有利的途径。值得注意的是 发现该催化剂对于通过β-氢化物(β-H)转移至金属而引起的降解相当稳定。用于TBP中间体转化为基于正棱锥(SP)的中间体的高活化势垒和较高的后期中间体能量提示催化剂对降解的稳定性。β-H转移的很高的阻隔性也不利于降解反应。的[Mo(N-2,6-Me 2 -C 6 H 3)(CHCMe 2 Ph)(IMes)(OCH(CF 3)2)+ B(Ar F)4 – ]之间反应的1 H NMR光谱1,7-辛二烯证实没有任何β-H转移。










































 京公网安备 11010802027423号
京公网安备 11010802027423号